Abstract
Primary parotid squamous cell carcinoma (pPSCC) is a rare salivary gland neoplasm. Due to the low incidence of pPSCC, there is a lack of clinical studies with large samples. The aim of this study was to identify prognostic factors and develop a nomogram for predicting overall survival (OS) and cancer specific survival (CSS) of pPSCC, with the goal of guiding clinical decision making. We identified eligible pPSCC patients from the Surveillance, Epidemiology, and End Results (SEER) database. All patients were randomly allocated to either the training or validation cohort in a 7:3 ratio. The X-tile software was utilized to determine the optimal cut-off values for age at diagnose, regional nodes examined, regional nodes positive, and tumor size, and changes continuous variables into categorical variables. Univariate and multivariate Cox regression analyses were used to identify independent prognostic factors. Based on the identified variables, two nomograms were developed and validated to predict the 1-, 3-, and 5-year OS and CSS of pPSCC. The accuracy of the prediction was evaluated using the C-index and calibration curve. Decision curve analysis (DCA) and receiver operating characteristic (ROC) were utilized to compare the nomogram with the American Joint Committee on Cancer (AJCC) stage system in order to assess its superiority. Furthermore, two risk stratification systems were established based on the constructed nomograms. From 2000 to 2019, a total of 2,187 pPSCC patients were screened from the SEER database. The incidence of pPSCC showed an overall upward trend, with the highest incidence in patients aged 71–80 years. The 495 patients with pPSCC ultimately identified from the SEER database were randomly allocated into a training cohort (n = 348) and a validation cohort (n = 147).Five independent prognostic variables were identified for OS, including age at diagnose, distant metastasis, AJCC stage, type of surgery, and tumor size. However, six independent prognostic variables were identified for CSS, with the addition of regional lymph node positivity as an additional variable. Nomograms of OS and CSS were established based on the results. In the training cohort and the validation cohort, the C-index of OS and CSS was 0.679, 0.677, 0.650 and 0.650 respectively. Calibration curve demonstrate that the predictions of 1-, 3-, and 5-year survival probability models for OS and CSS were generally consistent with actual observations in both the training cohort and the validation cohort. Our nomogram demonstrated a superior clinical net benefit compared to the AJCC 7th version, as indicated by DCA and ROC analysis. Additionally, patients were stratified into low-, middle-, and high-risk groups based on the nomogram risk score. The Kaplan-Meier curve demonstrated significant differences in survival among the three groups. In this study, new nomograms and risk classification systems were successfully developed to predict the 1-, 3-, and 5-year OS and CSS of pPSCC patients, which has good accuracy and superiority and can help doctors and patients make clinical decisions.
Keywords: Parotid gland, Squamous cell carcinoma, Prognostic factors, Nomograms, Overall survival, Cancer-specific survival
Subject terms: Surgical oncology, Oncology, Head and neck cancer
Introduction
Parotid gland cancers (PGCs) account for approximately 2% of head and neck malignancies, with an annual incidence ranging from 6 to 11 cases per one million people1–3. According to the current World Health Organization (WHO) classification, PGCs are categorized into 24 histological types4. Parotid squamous cell carcinoma (PSCC) is a rare and aggressive malignancy, which often presents at an advanced stage with nodal metastases5. The prognosis for PSCC is poor, as previously reported 5-year survival rates have been less than 50%6. PSCCs are typically the result of metastases from primary tumors located outside of the parotid gland7. The incidence of primary parotid squamous cell carcinoma (pPSCC) accounts for 1–3% of all parotid malignancies, and diagnosis of pPSCC relies on thorough exclusion of metastatic origin from any other primary malignancy8,9.
The current understanding of pPSCC is primarily based on small cohort studies and lacks evidence from large populations. The SEER database offers a natural advantage in its integration of tumor cases, providing accurate, comprehensive, and standardized case information. As a result of these population-based advantages, the SEER database facilitates the establishment of rare disease cohorts and effectively promotes research on rare diseases10.
Due to the heterogeneity of tumors, they should be treated as separate entities. The American Joint Committee on Cancer (AJCC) stage system is commonly utilized to predict the long-term survival of cancer patients11. However, AJCC stage does not account for individual patient factors such as age, gender, race, tumor site, surgery, radiotherapy and chemotherapy. As a result, it has certain limitations in making personalized predictions. Therefore, there is a need for an optimal model with improved predictive performance, and the nomogram proves to be a satisfactory tool for addressing these issues.
Nomograms are a visual representation of a complex mathematical formula. They utilize biological and clinical variables to graphically illustrate a statistical prognostic model, which in turn generates the probability of a clinical event for an individual12. Currently, the nomogram has been demonstrated as an effective method for predicting survival outcomes across various types of tumors13. However, to the best of our knowledge, there are still no reports of using a nomogram to predict the survival rate of pPSCC. Therefore, our study aims to establish a reasonable and effective nomogram to help predict overall survival (OS) and cancer-specific survival (CSS) in patients with pPSCC.
Patients and methods
Patients selection
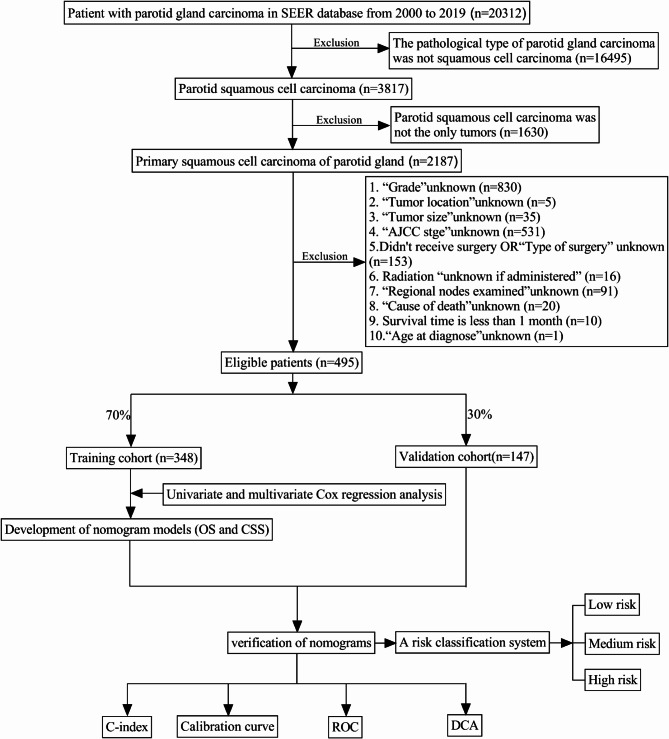
We utilized the SEER*Stat 8.4.3 program to access and retrieve patient data from the SEER 18 database. All patients diagnosed with PSCC (ICD-O-3 histologic types: 8052, 8070, 8071, 8072, 8073, 8074, 8075, 8083, 8084 and 8085) between 2000 and 2019 were identified. Exclusion criteria were as follows: (1) Parotid squamous cell carcinoma was not the only tumors; (2) patients who had no complete information; (3) Survival time is less than 1 month. Finally, a total of 495 patients were included in this study, and the selection flow chart is depicted in Fig. 1. Since public and anonymous data were utilized in accordance with ethical guidelines, neither informed consent nor approval from an ethics committee was deemed necessary.
Fig. 1.
Flowchart for selection patients with pPSCC.
Data collection
Clinical characteristics data, including age at diagnose, sex, race, tumor grade, laterality, distant metastasis status, AJCC stage, type of surgery, radiation status, chemotherapy status, regional nodes examined, regional nodes positive, tumor size, survival time, cause of death and survival status were collected from the SEER database for analysis. The X-tile software (Yale University, New Haven, Connecticut, USA) was utilized to determine the optimal cut-off values for age at diagnose, regional nodes examined, regional nodes positive, and tumor size (Fig. 2). The optimal cut-off value for age at diagnose was 77 years; the optimal cut-off value for regional nodes examined was 23; the optimal cut-off value for regional nodes positive was 4; the optimal cut-off value for tumor size was 28 mm. Given that the AJCC 6th edition of parotid gland tumor closely aligns with the AJCC 7th version, it is appropriate to convert the AJCC 6th version into the AJCC 7th version. This study ultimately adopts the AJCC 7th version. In this retrospective study, the primary research endpoints were OS and CSS. OS was defined as the time from the primary diagnosis to the date of death from any cause. CSS was defined as the time from the primary diagnosis to the date of death specifically attributed to pPSCC.
Fig. 2.
Estimation of the appropriate cutoff value for age at diagnose (A,B), regional nodes examined (C,D), regional nodes positive (E,F), tumor size (G,H), total risk score for OS (I,J) and total risk score for CSS (K,L) by X-tile analysis.
Development and validation of the prognostic nomogram
The patients were randomly divided into the training cohort and the validation cohort, consisting of 348 and 147 cases, respectively, in a ratio of 7:3. In the training cohort, univariate Cox regression analysis was conducted for each variable to identify potential prognostic risk factors. Subsequently, multivariate Cox regression analysis was performed based on these prognostic risk factors to determine independent prognostic factors in patients with pPSCC. Based on the independent prognostic factors, nomograms for OS and CSS at 1-, 3-, and 5-year were constructed using the “rms” package in R software. First, the concordance index (C-index) was utilized to assess discrimination. Additionally, calibration curves were constructed to evaluate the agreement between the predicted survival probability and the observed probability. Finally, DCA and ROC were used to compare the predictive value of our model with that of the AJCC stage. In contrast to ROC, DCA can also be utilized to assess the net clinical benefit of clinical outcomes at various probability thresholds.
Risk stratification
Based on the constructed nomogram, the total risk score for each patient was calculated. X-tile software was utilized to determine the optimal cut-off values for the total risk score. According to the optimal cut-off values, the patients were categorized into low-risk group, medium-risk group and high-risk group. Kaplan-Meier curve and log-rank test were employed to compare the difference in survival rate between different groups.
Statistical analysis
All statistical methods were conducted using SPSS 26.0 (IBM Corporation) and R software (version 4.3.3). The optimal cut-off values for all continuous variables were determined using the X-tile software. The Kaplan-Meier method was utilized to calculate the survival curves, and the disparity between the curves was assessed using the log-rank test. A p-value < 0.05 was considered significant.
Result
Baseline patient characteristics
From 2000 to 2019, a total of 2,187 pPSCC patients were screened from the SEER database. the incidence of pPSCC showed an overall upward trend, with the highest incidence in patients aged 71–80 years (Fig. 3). According to the inclusion and exclusion criteria, 495 patients with pPSCC were ultimately identified from the SEER database. The samples were then randomly divided into a training cohort (70%) and a validation cohort (30%). Clinical variables for patients in the two cohorts were compared, as detailed in Table 1. A chi-square test was conducted on the clinical information of both cohorts, yielding P values > 0.05. The results indicated no significant disparity in clinical information between the two groups.
Fig. 3.
(A) Annual patients of pPSCC from 2000 to 2019; (B) Incidence of pPSCC in patients of various age groups.
Table 1.
Clinicopathologic characteristics of the training cohort and the validation cohort.
| Variables | Total (n = 495) | Training cohort (n = 348) | Validation cohort (n = 147) | P value |
|---|---|---|---|---|
| Age at diagnose (years) | 0.806 | |||
| <77 | 287 (58.0%) | 203 (58.3%) | 84 (57.1%) | |
| ≥ 77 | 208 (42.0%) | 145 (41.7%) | 63 (42.9%) | |
| Sex | 0.215 | |||
| Male | 410 (82.8%) | 293 (84.2%) | 117 (79.6%) | |
| Female | 85 (17.2%) | 55 (15.8%) | 30 (20.4%) | |
| Race | 0.322 | |||
| Black | 9 (1.8%) | 8 (2.3%) | 1 (0.7%) | |
| White | 471 (95.2%) | 328 (94.3%) | 143 (97.3%) | |
| Other | 15 (3.0%) | 12 (3.4%) | 3 (2.0%) | |
| Grade | 0.600 | |||
| I–II | 210 (42.4%) | 145 (41.7%) | 65 (44.2%) | |
| III–IV | 285 (57.6%) | 203 (58.3%) | 82 (55.8%) | |
| Laterality | 0.434 | |||
| Left | 256 (51.7%) | 176 (50.6%) | 80 (54.4%) | |
| Right | 239 (48.3%) | 172 (49.4%) | 67 (45.6%) | |
| Distant metastasis | 0.101 | |||
| No | 483 (97.6%) | 337 (96.8%) | 146 (99.3%) | |
| Yes | 12 (2.4%) | 11 (3.2%) | 1 (0.7%) | |
| AJCC stage | 0.776 | |||
| I–II | 105 (21.2%) | 75 (21.6%) | 30 (20.4%) | |
| III–IV | 390 (78.8%) | 273 (78.4%) | 117 (79.6%) | |
| Type of surgery | 0.703 | |||
| Total or radical parotidectomy | 296 (59.8%) | 210 (60.3%) | 86 (58.5%) | |
| Local parotidectomy | 199 (40.2%) | 138 (39.7%) | 61 (41.5%) | |
| Radiotherapy | 0.675 | |||
| No | 148 (29.9%) | 106 (30.5%) | 42 (28.6%) | |
| Yes | 347 (70.1%) | 242 (69.5%) | 105 (71.4%) | |
| Chemotherapy | 0.379 | |||
| No | 383 (77.4%) | 273 (78.4%) | 110 (74.8%) | |
| Yes | 112 (22.6%) | 75 (21.6%) | 37 (25.2%) | |
| Regional nodes examined | 0.429 | |||
| < 23 | 326 (65.9%) | 233 (67.0%) | 93 (63.3%) | |
| ≥ 23 | 169 (34.1%) | 115 (33.0%) | 54 (36.7%) | |
| Regional nodes positive | 0.804 | |||
| < 4 | 411 (83.0%) | 288 (82.8%) | 123 (83.7%) | |
| ≥ 4 | 84 (17.0%) | 60 (17.2%) | 24 (16.3%) | |
| Tumor size (mm) | 0.681 | |||
| < 28 | 209 (42.2%) | 149 (42.8%) | 60 (40.8%) | |
| ≥ 28 | 286 (57.8%) | 199 (57.2%) | 87 (59.2%) | |
Cox regression analyses of variables for OS and CSS
The relationship between clinical features and prognosis of pPSCC was analyzed using univariate Cox regression. The results revealed that age at diagnose, distant metastasis, AJCC stage, type of surgery, regional nodes positive, and tumor size were significant prognostic risk factors for OS and CSS (P < 0.05), as presented in Table 2. Furthermore, a multivariate Cox regression analysis of clinical variables based on the identified risk factors demonstrated that age at diagnose, distant metastasis, AJCC stage, type of surgery, and tumor size were independent prognostic factors for OS in patients with pPSCC. Age at diagnose, distant metastasis, AJCC stage, type of surgery, regional nodes positive, and tumor size were also found to be independent prognostic factors for CSS as shown in Table 3. According to the results of multivariate analysis, patients with younger age of diagnose, absence of distant metastasis, lower AJCC stage, local parotidectomy, and smaller tumor size demonstrated improved OS outcomes. Additionally, based on the results of multivariate analysis, patients with younger age of diagnose, no distant metastasis, lower AJCC stage, local parotidectomy, fewer positive regional nodes and smaller tumor size showed improved CSS outcomes (Fig. 4).
Table 2.
Univariate cox regression model in the training cohort.
| Variables | OS | P value | CSS | P value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age at diagnose (years) | ||||
| < 77 | Reference | Reference | ||
| ≥ 77 | 2.473 (1.907, 3.206) | 0.000 | 1.450 (1.037, 2.027) | 0.030 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.898 (0.628, 1.286) | 0.559 | 1.017 (0.651, 1.590) | 0.940 |
| Race | ||||
| Black | Reference | Reference | ||
| White | 1.347 (0.555, 3.270) | 0.510 | 0.752 (0.308, 1.838) | 0.532 |
| Other | 0.493 (0.132, 1.836) | 0.292 | 0.364 (0.087, 1.524) | 0.167 |
| Grade | ||||
| I–II | Reference | Reference | ||
| III–IV | 1.036 (0.800, 1.342) | 0.788 | 1.106 (0.791, 1.548) | 0.555 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | 1.231 (0.954, 1.588) | 0.109 | 1.118 (0.805, 1.551) | 0.507 |
| Distant metastasis | ||||
| No | ||||
| Yes | 2.706 (1.433, 5.110) | 0.002 | 3.979 (2.019, 7.844) | 0.000 |
| AJCC stage | ||||
| I–II | Reference | Reference | ||
| III–IV | 1.867 (1.319, 2.642) | 0.000 | 2.559 (1.541, 4.248) | 0.000 |
| Type of surgery | ||||
| Total or radical parotidectomy | Reference | Reference | ||
| Local parotidectomy | 0.625 (0.477, 0.817) | 0.001 | 0.560 (0.392, 0.799) | 0.001 |
| Radiotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.771 (0.588, 1.011) | 0.060 | 0.939 (0.655, 1.347) | 0.733 |
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 1.103 (0.816, 1.492) | 0.522 | 1.319 (0.910, 1.912) | 0.143 |
| Regional nodes examined | ||||
| < 23 | Reference | Reference | ||
| ≥ 23 | 1.230 (0.942, 1.605) | 0.128 | 1.256 (0.893, 1.766) | 0.190 |
| Regional nodes positive | ||||
| < 4 | Reference | Reference | ||
| ≥ 4 | 1.574 (1.146, 2.163) | 0.005 | 2.125 (1.465, 3.082) | 0.000 |
| Tumor size (mm) | ||||
| <28 | Reference | Reference | ||
| ≥ 28 | 1.878 (1.438, 2.452) | 0.000 | 1.879 (1.329, 2.656) | 0.000 |
Table 3.
Multivariate cox regression model in the training cohort.
| Variables | OS | P value | CSS | P value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age at diagnose (years) | ||||
| < 77 | Reference | Reference | ||
| ≥ 77 | 2.591 (1.993, 3.367) | 0.000 | 1.515 (1.081, 2.123) | 0.016 |
| Distant metastasis | ||||
| No | Reference | Reference | ||
| Yes | 3.134 (1.614, 6.084) | 0.001 | 4.060 (1.989, 8.287) | 0.000 |
| AJCC stage | ||||
| I–II | Reference | Reference | ||
| III–IV | 1.543 (1.070, 2.226) | 0.020 | 1.833 (1.075, 3.125) | 0.026 |
| Type of surgery | ||||
| Total or radical parotidectomy | Reference | Reference | ||
| Local parotidectomy | 0.715 (0.542, 0.944) | 0.018 | 0.624 (0.432, 0.901) | 0.012 |
| Regional nodes positive | ||||
| < 4 | Reference | Reference | ||
| ≥ 4 | 1.152 (0.827, 1.605) | 0.404 | 1.518 (1.026, 2.246) | 0.037 |
| Tumor size (mm) | ||||
| < 28 | Reference | Reference | ||
| ≥ 28 | 1.726 (1.309, 2.277) | 0.000 | 1.523 (1.062, 2.183) | 0.022 |
Fig. 4.
OS and CSS for the pPSCC patients using Kaplan–Meier analysis and log-rank test. (A) OS among pPSCC patients based on age at diagnose. (B) OS among pPSCC patients based on distant metastasis. (C) OS among pPSCC patients based on AJCC stage. (D) OS among pPSCC patients based on type of surgery. (E) OS among pPSCC patients based on tumor size. (F) CSS among pPSCC patients based on age at diagnose. (G) CSS among pPSCC patients based on distant metastasis. (H) CSS among pPSCC patients based on AJCC stage. (I) CSS among pPSCC patients based on type of surgery. (J) CSS among pPSCC patients based on regional nodes positive. (K) CSS among pPSCC patients based on tumor size.
Construction of the prognostic nomogram
The nomogram was constructed based on the identification of independent prognostic factors through multifactor Cox regression analysis, with the aim of predicting 1-, 3-, and 5-year OS (Fig. 5A) as well as CSS (Fig. 5B). The Nomogram is capable of converting the correlation coefficient of each variable into scores ranging from 0 to 100. The total score is calculated by summing up the scores of each variable. By drawing straight lines from the total score scale, one can obtain the probabilities of survival at 1-, 3-, and 5-year. The nomogram for OS demonstrates that age at diagnose and distant metastasis exert the most significant impact on the survival outcome of patients with pPSCC. Distant metastasis and AJCC stage are key factors influencing the prognosis of patients in the CSS nomogram. In comparison to the OS nomogram, the influence of age at diagnosis on patient prognosis is notably diminished in the CSS nomogram.
Fig. 5.
Nomograms for predicting 1-,3- and 5-year OS (A) and CSS (B) in patients with pPSCC (training cohort).
Validation of the prognostic nomogram
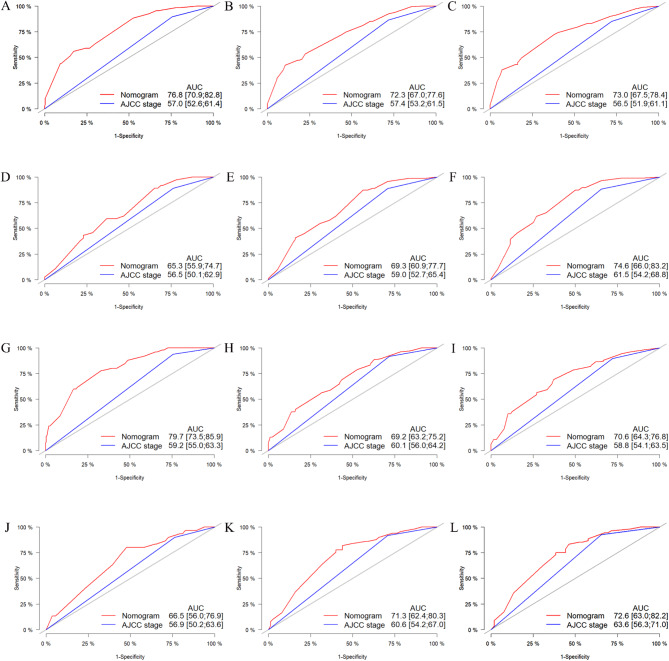
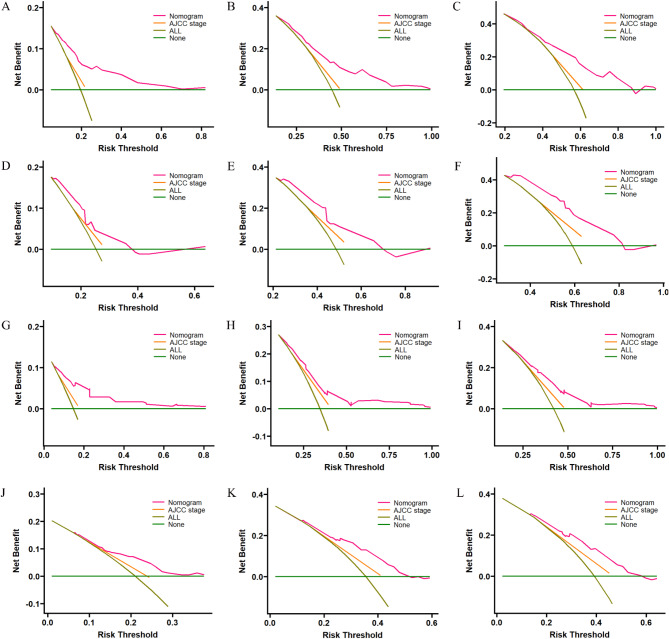
The accuracy and superiority of the nomogram were verified through various methods, including internal and external validation. Internal validation was conducted using the training cohort, while external validation was performed using the validation cohort. First, in the training cohort, the C-index for OS and CSS was 0.679 (95% CI, 0.645–0.713) and 0.677 (95% CI, 0.633–0.721), respectively. Similarly, in the validation cohort, the corresponding C-index values were 0.650 (95% CI, 0.594–0.706) and 0.650 (95% CI, 0.580–0.720). Indicates that the nomogram demonstrates a good degree of discrimination. Then, the calibration curve and ROC curve were generated with data from the training and validation cohorts. Calibration curve demonstrate that the predictions of 1-, 3-, and 5-year survival probability models for OS and CSS were generally consistent with actual observations in both the training cohort and the validation cohort (Fig. 6). ROC analysis conducted in the training cohort demonstrated AUC values of 76.8, 72.3, and 73.0 for 1-year, 3-year, and 5-year OS, respectively. Additionally, the AUC values for CSS was determined to be 79.7, 69.2, and 70.6 at different time points, both of which exceeded the corresponding AJCC stage. In the validation cohort, similar results were observed with AUC values of 65.3, 69.3, and 74.6 for OS at different time points, and AUC values of 66.5, 71.3, and 72.6 for CSS, all of which exceeded those of the corresponding AJCC stage as illustrated in Fig. 7. These findings indicate that the nomogram exhibited superior discriminatory ability compared to AJCC stage. Furthermore, we constructed DCA curves to assess the predictive performance of the nomogram compared to traditional AJCC stage. The findings indicated that both models provided significantly greater benefit than AJCC stage (Fig. 8).
Fig. 6.
The calibration plots of the nomogram predicting 1-, 3- and 5-year OS in the training cohort (A–C) and validation cohort (D–F). The calibration plots of the nomogram predicting 1-, 3- and 5-year CSS in the training cohort (G–I) and validation cohort (J–L).
Fig. 7.
ROC curves for the 8th version of the AJCC stage system and nomogram were used to predict 1-, 3-, and 5-year OS in both the training cohort (A–C) and validation cohort (D–F), as well as 1-, 3-, and 5-year CSS in both the training cohort (G–I) and validation cohort (J–L).
Fig. 8.
DCA curves for the 8th version of the AJCC stage system and nomogram were used to predict 1-, 3-, and 5-year OS in both the training cohort (A–C) and validation cohort (D–F), as well as 1-, 3-, and 5-year CSS in both the training cohort (G–I) and validation cohort (J–L).
Risk stratification of OS and CSS
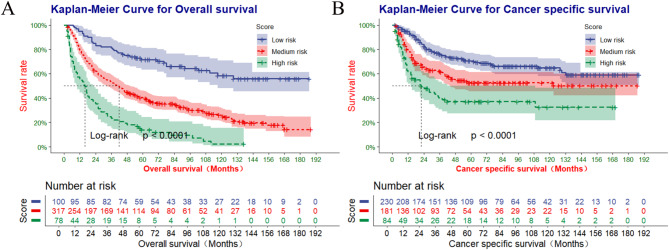
The total scores for all patients diagnosed with pPSCC were calculated using the nomogram, and the optimal cut-off values for OS and CSS were determined using X-tile software. The optimal cut-off values for OS were identified as 38.5 and 163.8, while the optimal cut-off values for CSS were found to be 104.9 and 159.1 (Fig. 2I-L). Primary PSCC patients are stratified into high-risk (score > 163.8), medium-risk (38.5 < score ≤ 163.8), and low-risk (score ≤ 38.5) categories for OS. Additionally, pPSCC patients are categorized as high-risk (score > 159.1), medium-risk (104.9 < score ≤ 159.1), and low-risk (score ≤ 104.9) for CSS. According to risk stratification, Kaplan-Meier survival curves were generated for all patients diagnosed with pPSCC, as depicted in Fig. 9. The 1-, 3-, and 5-year OS rates were 54.6%, 23.4%, and 13.9% in the high-risk group; 79.4%,53.1%, and 40.5% in the medium-risk group; and 95.0%, 81.0%, and 71.6% in the low-risk group, respectively. The corresponding CSS rates were also calculated for each risk group (61.5%,40.3%,and 37.2%;78.8%,62.3%,and52.7%;94.7%,76.0%,and 70.6%).There was a statistically significant difference in survival outcomes among the three groups (P < 0.001).
Fig. 9.
The Kaplan-Meier survival curves of OS (A) and the Kaplan-Meier survival curves of CSS (B) by the risk score calculated by the nomogram.
Discussion
Primary PSCC is a rare tumor, and there are fewer clinical research data and literature available compared to other tumors. Currently, the primary criteria for evaluating the prognosis of pPSCC are based on the AJCC stage system. However, the AJCC stage system still has its limitations14. Patients with the same AJCC stage but different survival outcomes will be categorized into the same disease stage, leading to heterogeneity in prognosis. Compared to the AJCC stage system, the nomogram has been demonstrated to be more accurate in predicting prognosis for various types of cancer13,15. For the first time, this study has developed a nomogram to predict the prognosis of patients with pPSCC.
Based on the SEER database, a total of 495 patients with pPSCC were included in the study. This study found that age at diagnosis, distant metastasis, AJCC stage, type of surgery, and tumor size were independent predictors of OS in patients with pPSCC. Additionally, age at diagnose, distant metastasis, AJCC stage, type of surgery, regional nodes positive, and tumor size were identified as independent predictors of CSS in pPSCC patients. Based on the aforementioned variables, nomograms were developed and validated to accurately predict OS and CSS for 1-, 3-, and 5-year with superior performance compared to the AJCC stage system. Furthermore, a successful risk stratification has been established based on the total risk score determined by the nomogram.
We divided the patients into two groups and determined 77 years as the optimal age cutoff via X-tile software. The findings of this study indicate that advancing age is linked to poorer survival outcomes. This finding is in line with the outcomes of prior research studies16–18. This could be attributed to the fact that elderly patients often have multiple underlying conditions such as diabetes and high blood pressure, which are linked to a poorer prognosis19.
It is widely recognized that the progression and stage of tumors can have a significant impact on a patient’s prognosis. The results of this study indicate that distant metastasis serves as an independent prognostic factor in patients diagnosed with pPSCC. Previous research has indicated that patients diagnosed with pPSCC experience a poorer prognosis when they occur distant metastases9. These results are consistent with our own. At the same time, our findings suggest that tumor stage is an independent prognostic factor in patients with pPSCC. This finding is in line with the Xiao’s study, which also identified variations in tumor stage and prognosis among patients with pPSCC20.
A high rate of regional lymph node metastasis is a significant clinical feature of pPSCC and is closely associated with the prognosis of this condition21–23. The identical results were discovered in this study. Additionally, we discovered that a fewer number of positive regional nodes was associated with improved CSS.
The impact of tumor size on the prognosis of patients with pPSCC remains a topic of controversy. In this study, a tumor size greater than 28 mm was identified as an independent prognostic factor for patients diagnosed with pPSCC. This result is in line with the findings of Chen’s8. However, an analysis of 24 patients with pPSCC has indicated that tumor size does not serve as prognostic factor for this type of cancer24. This may be attributed to the small sample size of this study, which increases the likelihood of obtaining false negative results.
Surgery continues to be the preferred treatment for patients diagnosed with pPSCC. Chen’s research findings indicate that surgical intervention plays a crucial role in determining the prognosis of patients diagnosed with pPSCC9. However, other studies have not found a significant association between surgery and patients prognosis25. In this study, surgery is identified as a significant risk factor impacting the prognosis of pPSCC. Furthermore, we observed a statistically significant difference between local parotidectomy and total or radical parotidectomy. The prognosis following local parotidectomy is superior to that of total or radical parotidectomy.
This study possesses numerous advantages. Firstly, To the best of our knowledge, this is the first predictive nomogram developed for patients with pPSCC, and it demonstrates good predictive performance. Furthermore, this study ultimately involved 495 patients, demonstrating a strong level of representativeness. Finally, A risk stratification system was successfully established based on the nomogram, in order to personalize treatment for individual patients.
Naturally, there are some limitations to this study. Firstly, it is important to note that this study is retrospective in nature. As a result, it is susceptible to biases stemming from the retrospective design and follow-up compliance. In the future, prospective studies will be required to validate these findings. Furthermore, the SEER database does not contain detailed information on radiotherapy, chemotherapy, and surgery. Finally, this study only utilized internal validation, which may result in overfitting of the model. This question will be explored in our upcoming research.
Conclusions
In this study, we investigated prognostic factors in patients with pPSCC. The results revealed that age at diagnose, distant metastasis, AJCC stage, type of surgery, and tumor size were identified as independent risk factors for OS. Similarly, age at diagnose, distant metastasis, AJCC stage, type of surgery, regional nodes positive, and tumor size were also found to be independent risk factors for CSS. We constructed two nomograms to predict OS and CSS in patients with pPSCC. Following internal validation, the nomogram demonstrated high accuracy and reliability. This predictive tool can assist healthcare providers in anticipating the prognosis of patients with pPSCC and devising individualized treatment plans accordingly.
Acknowledgements
The authors would like to thank all members of the SEER Program for their contributions to the SEER database.
Author contributions
X.L., J.X., Y.W.: Conception and design: J.X., F.D., Y.W.: Administrative support: X.L., J.X., Y.W.: Provision of study materials or patients X.L.; J.X., X.W., Y.T., Q.L., J.W.: Collection and assembly of data: Data analysis and interpretation: All authors; Manuscript writing: All authors; All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work had the following financial supports: Gansu Province Health Industry Research Project (No. GSWSKY2023-30); Science and Technology Project of Gansu Province (No. 24JRRA695); Lanzhou Science and Technology Development Plan Project (No. 2022-3-26).
Data availability
The dataset presented in this study is available in the SEER database (http://seer.cancer.gov/seerstat).
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study is based on data from the SEER database and does not require ethical approval.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xudong Liu and Jincai Xue have contributed equally to this work.
References
- 1.Shi, X. et al. Anatomic extent of lymph node metastases as an independent prognosticator in node-positive major salivary gland carcinoma: A study of the US SEER database and a Chinese multicenter cohort. Eur. J. Surg. Oncol.45(11), 2143–2150. 10.1016/j.ejso.2019.06.029 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Fang, Q., Liu, F. & Seng, D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manag. Res.11, 1081–1085. 10.2147/CMAR.S192788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park, Y. M. & Koh, Y. W. Current issues in treatment of parotid gland cancer and advanced surgical technique of robotic parotidectomy. Curr. Oncol. Rep.24(2), 203–208. 10.1007/s11912-021-01167-y (2022). [DOI] [PubMed] [Google Scholar]
- 4.Inaka, Y. et al. Symptoms and signs of parotid tumors and their value for diagnosis and prognosis: A 20-year review at a single institution. Int. J. Clin. Oncol.26(7), 1170–1178. 10.1007/s10147-021-01901-3 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Nykänen, N. et al. Ex vivo drug screening informed targeted therapy for metastatic parotid squamous cell carcinoma. Front. Oncol.11, 735820. 10.3389/fonc.2021.735820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfisterer, M. J. et al. Squamous cell carcinoma of the parotid gland: A population-based analysis of 2545 cases. Am. J. Otolaryngol.35(4), 469–475. 10.1016/j.amjoto.2014.03.003 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Franzen, A., Lieder, A., Guenzel, T. & Buchali, A. The heterogenicity of parotid gland squamous cell carcinoma: A study of 49 patients. In Vivo.33(6), 2001–2006. 10.21873/invivo.11696 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horáková, Z. et al. Primary squamous cell carcinoma of the parotid gland: Study and review of the literature. In Vivo38(1), 358–364. 10.21873/invivo.13446 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, M. M., Roman, S. A., Sosa, J. A. & Judson, B. L. Prognostic factors for squamous cell cancer of the parotid gland: An analysis of 2104 patients. Head Neck37(1), 1–7. 10.1002/hed.23566 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Che, W. Q. et al. How to use the surveillance, epidemiology, and end results (SEER) data: Research design and methodology. Mil Med. Res.10(1), 50. 10.1186/s40779-023-00488-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gospodarowicz, M. K. et al. The process for continuous improvement of the TNM classification. Cancer100(1), 1–5. 10.1002/cncr.11898 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: More than meets the eye. Lancet Oncol.16(4), e173–e180. 10.1016/S1470-2045(14)71116-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, Y. Y. et al. New nomograms to predict overall and cancer-specific survival of angiosarcoma. Cancer Med.11(1), 74–85. 10.1002/cam4.4425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin, M. B. et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J. Clin.67(2), 93–99. 10.3322/caac.21388 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Qi, W. et al. Establishment and validation of nomogram models for overall survival and cancer-specific survival in spindle cell sarcoma patients. Sci. Rep.13(1), 23018. 10.1038/s41598-023-50401-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shemen, L. J., Huvos, A. G. & Spiro, R. H. Squamous cell carcinoma of salivary gland origin. Head Neck Surg.9(4), 235–240. 10.1002/hed.2890090407 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Ying, Y. L., Johnson, J. T. & Myers, E. N. Squamous cell carcinoma of the parotid gland. Head Neck28(7), 626–632. 10.1002/hed.20360 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Jering, M. et al. Cancer-specific and overall survival of patients with primary and metastatic malignancies of the parotid gland—A retrospective study. J. Craniomaxillofac. Surg.50(5), 456–461. 10.1016/j.jcms.2022.03.001 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Sun, H. et al. Construction and validation of prognostic nomograms for elderly patients with metastatic non-small cell lung cancer. Clin. Respir J.16(5), 380–393. 10.1111/crj.13491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao, M., Liu, J., You, Y., Yang, X. & Wang, Y. Primary squamous cell carcinoma of the parotid gland: Clinicopathological characteristics, treatment, and prognosis. Int. J. Oral Maxillofac. Surg.50(2), 151–157. 10.1016/j.ijom.2020.06.010 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Gaughan, R. K., Olsen, K. D. & Lewis, J. E. Primary squamous cell carcinoma of the parotid gland. Arch. Otolaryngol. Head Neck Surg.118(8), 798–801. 10.1001/archotol.1992.01880080020006 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Gallo, O. et al. Risk factors for distant metastases from carcinoma of the parotid gland. Cancer80(5), 844–851 (1997). [PubMed] [Google Scholar]
- 23.Spiro, R. H., Huvos, A. G. & Strong, E. W. Cancer of the parotid gland. A clinicopathologic study of 288 primary cases. Am. J. Surg.130(4), 452–459. 10.1016/0002-9610(75)90483-3 (1975). [DOI] [PubMed] [Google Scholar]
- 24.Liu, J., Tang, P. Z., Xu, Z. G., Liu, S. Y. & Wang, X. L. Diagnosis and management of primary squamous cell carcinoma of parotid gland. Chin. Arch. Otolaryngol. Head Neck Surg.17(04), 187–189. 10.16066/j.1672-7002.2010.04.025 (2010). [Google Scholar]
- 25.Qiu, W. et al. The role of postoperative radiotherapy and prognostic model in primary squamous cell carcinoma of parotid gland. Front. Oncol.10, 618564. 10.3389/fonc.2020.618564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset presented in this study is available in the SEER database (http://seer.cancer.gov/seerstat).