Abstract
Background
Dementia clinics traditionally focus on diagnosis and post-diagnostic care. Awareness is increasing that attention to risk factors and their prevention also forms a key part of dementia management.
Objectives
To describe our Bristol Brain Health clinic including 1) Clinical pathway 2) Patient population 3) Patient experience 4) Evaluation in line with published gold standards.
Design/ setting
Observational, (longitudinal/retrospective) clinical cohort study of patients attending the North Bristol NHS Trust's Brain Health Service.
Participants
One-hundred and ten patients with mild cognitive disorders attending clinic between 2017- 2023.
Measurements
We collected data from medical records including clinical assessments, cerebrospinal fluid (CSF) for biomarkers of Alzheimer's Disease (AD), and a lifestyle questionnaire. Descriptive statistics were performed and a clinic evaluation was carried out using recommendations from The European Task Force for Brain Health Services.
Results
Average age was 63.9 years (SD: 11.2). 74 patients were male (62.8 %). The mean baseline Montreal Cognitive Assessment (MoCA) score was 24.4 (SD: 3.6). 73 patients (66.4 %) received a preventative lifestyle intervention with a review of risk and protective factors for dementia, and development of a bespoke risk reduction plan. Commonly identified risk factors; low mood; n = 61 (55.5 %), hypertension; n = 54 (49.1 %), high cholesterol; n = 42 (47.3 %), and hearing loss; n = 44 (40 %). CSF testing for AD was carried out in 38 individuals and was positive in 17 cases. At last review, one fifth of patients had progressed to dementia. Most common diagnoses; AD; n = 22 (20 %), Functional Cognitive Disorder; n = 16 (14.6 %), Vascular; n = 8 (7.3 %). Patient feedback was good, with all responders recommending the clinic and more than three-quarters of patients being ‘extremely likely” to. Clinic evaluation highlighted ‘Risk Assessment’ and ‘Personalised Intervention’ as brain health pillar strengths. ‘Cognitive Enhancement’ was an area for further development.
Conclusions
Our patients had access to a range of cutting-edge, diagnostic assessments, in addition to a preventative lifestyle intervention. Our population had a high rate of dementia risk factors and a heterogeneous range of diagnoses. CSF biomarker testing was helpful for differentiating between those with early AD, and others with a multi-factorial presentation. The attendance rates for our preventative intervention suggests patients are receptive to taking a proactive approach to managing risk. This population merits further investigation and continued targeting with preventative measures.
Keywords: Brain Health, Dementia risk, Preventative lifestyle intervention, CSF biomarkers, Alzheimer's disease
1. Background
Dementia is one of the leading UK health demands and cases are forecast to rise to 1.4 million by 2040 [1]. The most common cause of Dementia in the UK is Alzheimer's Disease (AD) [2]. Although Alzheimer's Disease (AD) pathology can be detected in people with no identifiable symptoms, the earliest symptomatic stage described is Subjective Cognitive Impairment (SCI) – a self-reported decline in cognitive functioning in the absence of objective deficits on standard neuropsychological tests, with intact activities of daily living (ADLs) [3].This may progress to Mild Cognitive Impairment (MCI), which is characterized by objective memory deficits >1.5 standard deviations below normative values on memory tests, and unimpaired activities of daily living (ADL) [4,5]. Patients often present to memory clinics at these early stages of the disease, prior to meeting criteria for dementia.
Historically, there have been limited therapeutic avenues available to individuals at these earlier stages in the disease process [6]. Most memory clinics have been primarily designed for people with significant cognitive impairment [7]. Those with SCI and MCI are, however, at increased risk of developing dementia compared to the general population [[3], [4], [5], [6], [7], [8]]. They are also often more likely to have insight into their difficulties than patients with dementia [9,10], and are generally motivated to seek support with risk reduction and improving their cognition [6].
It is estimated that up to 45.3 % of dementia cases could be prevented by leading healthier lifestyles, with 14 modifiable risk factors identified [11]. There is also growing evidence that lifestyle interventions can improve cognition. The FINGER Trial demonstrated benefits of a bespoke, multi-modal lifestyle intervention in slowing cognitive decline in elderly adults at risk of developing dementia [12]. Targeting individuals at these earlier stages in the disease process could be important for improving cognition, reducing the risk of dementia, and improving quality of life.
Prodromal stages of AD are now becoming the focus for a new generation of disease modifying therapies (DMTs) such as monoclonal antibodies, with the expectation that these treatments will have greatest impact at the earliest stages of the disease process prior to irreversible cerebral loss [13,14]. This places more emphasis on the need for clinical services focused on early diagnosis and intervention, facilitated by the advent of biomarkers [15,16].
Established in September 2017, The Bristol Brain Health Clinic was one of the first to be set up in the UK. This clinical service was designed for patients with mild cognitive syndromes including MCI and SCI. The purpose of the clinic was to offer individual risk assessment, early diagnosis, preventative interventions, and research opportunities.
As brain health clinics (BHCs) are a relatively new phenomenon, the European Task Force for Brain Health Services recently published guidelines to support clinicians with service set-up [6]. Evaluation of these novel services is important to assess cost effectiveness and clinical impact.
1.1. Project objectives
-
1.
Describe the clinic structure and interventions.
-
2.
Summarise our patient population including demographics, co-morbidities, and risk factors for dementia, including an exploratory analysis of differences between people who were positive and negative for AD biomarkers.
-
3.
Assess patient experience through use of questionnaires.
-
4.
Evaluate the clinic in line with recommendations from The European Task Force for Brain Health Services, highlighting areas for future development.
2. Methods
2.1. Study design and sample selection
This study was an observational, clinical cohort study (longitudinal/retrospective). All patients attending at least one appointment in the BHC between September 2017 - August 2023 were included in the study. Patients were retrospectively identified from clinical records.
2.2. Data source
This study utilised a range of data collected as part of routine clinical assessments. Relevant data were extracted from NHS clinical systems including Careflow Electronic Patient Record (Careflow EPR), ICE (Integrated Clinical Environment), EDMS (Electronic Data Management Service), and Connecting Care (GP Records).
2.3. Study measurements
Study measures included participant characteristics such as demographics, cognitive performance, prevalence of medical co-morbidities, risk and protective factors for dementia, and CSF biomarker results. Patient outcomes such as diagnosis and follow-up time in clinic were also reported, in addition to patient feedback and clinic evaluation.
2.4. Data analysis
Descriptive statistics were performed in Microsoft Excel (Version 2407). Means, standard deviations (SDs) and ranges were used to represent continuous measures. Counts and percentages were provided for categorical variables.
3. Brain health clinic and interventions
3.1. Cognitive clinic pathway
Primary mode of entry to the BHC was with initial review from a Consultant Neurologist in the NBT Cognitive Disorders Clinic (CDC). The CDC is a specialist outpatient diagnostic service for neurodegenerative diseases and complex cognitive conditions with Consultant Neurologist, Nurse Specialist, and Neuropsychology review. The CDC receives referrals from primary, secondary and tertiary care. Patient evaluation within the clinic includes serological testing, neuroimaging, and access to CSF testing for biomarkers of neurodegenerative disease. Patients have a baseline cognitive assessment with The Montreal Cognitive Assessment (a multi-domain cognitive screening tool used in clinic, with a maximum possible score of 30) [17] and a neuropsychometry battery devised by one of our Consultant Neuropsychologists.
Since 2022, patients and an informant (a carer, friend or relative) completed clinical questionnaire packs sent out by post prior to their appointment. Questionnaires measured sleep quality, functioning with ADLs, neuropsychiatric symptoms and subjective perception of memory difficulties. Sleep questionnaires included the Pittsburgh Sleep Quality Index (PSQI) which measures 7 domains pertinent to sleep quality [18], and the Epworth Sleepiness Scale (ESS), which measures severity of daytime sleepiness possibly indicative of an underlying sleep disorder [19]. A score of ≥5 on the PSQI is indicative of poor sleep quality [18]. A score of >11 on the ESS is suggestive of levels of daytime sleepiness possibly indicative of an underlying sleep disorder [19].
Cerebrospinal fluid (CSF) biomarker testing for AD was offered where clinically appropriate. CSF biomarkers were collected as per standard lumbar puncture procedure conducted in accordance with gold standard clinical protocol. Analysis was carried out at the Neuroimmunology and CSF Laboratory at University College London Hospitals (UCLH). See supplementary information for further details of all clinical investigations [20], [21].
3.2. Research
The BHC is an active research site running several commercial and academic trials. Since 2022, all patients attending the CDC/ BHC were invited to participate in DREAMS-AD [22], an observational study using routinely collected clinical data to primarily explore relationships between sleep and memory. A study invitation letter and participant information sheet were enclosed in the questionnaire pack. If willing to participate, patients were consented to the study at their clinic appointment. Additional research opportunities were also discussed if interested, including opportunities to take part in Patient and Public Involvement (PPI), which is the foundation of our research programme.
4. The brain health clinic
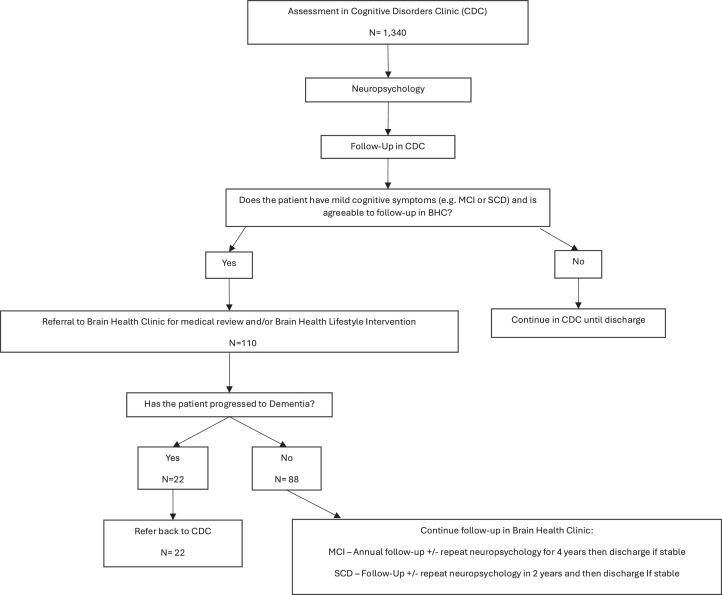
Patients with mild cognitive symptoms (e.g.: subjective cognitive impairment (SCI) but no objective cognitive impairment, or mild cognitive impairment (MCI), were offered the opportunity for continued assessment and follow up within the Brain Health Clinic. One patient with dementia was exceptionally referred to the BHC for an interest in research. This outlier has been included in the cohort for completeness. See Fig. 1 for further details of patient pathway.
Fig. 1.
Patient pathway in the NBT Cognitive Disorders Clinic and Brain Health Clinic.
Note. The number of assessments in CDC (N= 1340) corresponds to the number of new patients seen in clinic between 2017 and 2023, thereby excluding DNAs and cancellations.
4.1. Clinician review in brain health clinic
Patients attended a 40-minute appointment with a medical clinician to review cognitive symptoms and any changes in functioning in basic and instrumental ADLs [23]. Patients were encouraged to bring an informant to their medical appointment who could provide collateral history. Patients were actively screened for affective disorders, sleep disorders, cardiovascular risk factors and other relevant factors. Medication lists were also checked for potential cognitive side effects. Following review, a referral could be made to other services to aid management of co-morbid conditions if considered necessary e.g.: Sleep Clinic, Community Mental Health Team, Primary Care review (e.g. For management of cardiovascular risk factors). Time to follow up varied between patients depending on clinical need (see Fig. 1).
4.2. Brain health lifestyle sessions
Patients were offered a one hour, 1:1 appointment with the Brain Health Lifestyle Clinician. This was an evidence-based lifestyle intervention informed by risk factors outlined in the 2020 Lancet Commission for Dementia Prevention [24]. A questionnaire was used to record dementia risk and protective factors for each patient which included smoking, alcohol consumption, nutrition, weight management, exercise, sleep, cognitive and social stimulation, mental wellbeing, blood pressure, cholesterol levels and diabetes risk. This information was used to formulate a bespoke risk reduction plan with clear actionable steps aimed at optimising brain health. Relevant supplementary resources were also provided. All patients were offered a one year follow up to review their progress.
4.3. Assessments
The majority of patients had standardised clinical assessment at first presentation to the Cognitive Clinic. However, the BHC Clinician could request further serological, neuroimaging and neuropsychological testing as clinical need required.
Patient feedback was collected following their BHC appointment with a questionnaire developed in house. Feedback was not collected continuously throughout the 6-year period.
5. Results
5.1. Brain health clinic patient demographics
A total of 110 patients were seen in the BHC over six years. Group demographics are detailed in Table 1.
Table 1.
Brain health clinic population – baseline demographics (n = 110).
| Demographics | N (%) | Mean (SD) | Range | Missing Data N (%) |
|---|---|---|---|---|
| Age (Years) | 63.9 (11.2) | 37 – 87 | ||
| Sex | ||||
| Male | 74 (67.3) | |||
| Female | 36 (32.73) | |||
| Education | 13.2 (2.6) | 9 - 20 | 1 (0.9) | |
| Ethnicity | 32 (29.1) | |||
| White British | 72 (65.5) | |||
| White – Any Other Background | 1 (0.9) | |||
| White- Irish | 1 (0.9) | |||
| White – Any Other White Background | 1 (0.9) | |||
| Asian or Asian British – Bangladeshi | 1 (0.9) | |||
| Asian or Asian British - Indian | 1 (0.9) | |||
| Black or Black British Caribbean | 1 (0.9) | |||
| MoCA Score | 24.4 (3.6) | 15–30 | 2 (1.8) |
Note. Age at first Brain Health Clinic appointment, and MoCA score closest to first BHC appointment. Reasons for 2 excluded MoCAs 1) Telephone MoCA administered during Covid-19 Pandemic (total score out of 22 instead of 30) 2) Unable to locate score on medical records.
6. Investigations performed
6.1. Neuroimaging
Neuroimaging was conducted in 109 (99.1 %) patients. One patient self-discharged before a scan could take place. Patients underwent the following neuroimaging; Magnetic Resonance Imaging (MRI); n = 90 (81.8 %), repeat MRI to determine the extent/ nature of any progression, n = 26 (23.6 %), Computed Tomography (CT); n = 64 (58.2 %), repeat CT; n = 27 (24.6 %); Dopamine Active Transporter (DaT); n = 10 (9.1 %), Single-Photon Emission Computed Tomography (SPECT); n = 7 (6.4 %), combination of different types of scans; n = 54 (49.1 %).
6.2. MoCA
All patients completed at least one MoCA [25], although 2 patients were excluded from analyses due to reasons stated above. The mean group score of 24.4 falls within the MCI range [17,26] (see Table 1). It is important to note that non-native English speakers, or patients with an underlying diagnosis of a functional cognitive disorder, could return lower MoCA scores despite preserved day-to-day activities of daily living.
6.3. Neuropsychometry
Almost all of our patients completed at least once neuropsychological battery (n = 108, 98.2 %), and 35 (31.8 %) had repeat neuropsychological assessments.
6.4. CSF biomarkers
CSF biomarker testing was conducted in 38 (34.6 %) patients to understand the underlying cause of cognitive difficulties, and for the purposes of diagnostic exclusion. Of these individuals, 17 (44.7 %) had positive biomarkers in keeping with AD.
6.5. Brain health lifestyle sessions
Brain Health Lifestyle sessions were attended by 73 (66.4 %) patients. Sessions were introduced in November 2019, therefore patients seen in the early years of clinic did not have this option available to them. Leaflets on reducing the risk of dementia were provided up until this point instead. Of the patients who did not have the lifestyle intervention (n = 37, 33.6 %), the reasons for this were as follows: Discharged before lifestyle intervention was available; n = 13 (35.1 %), declined intervention; n = 4 (10.8 %), agreed to attend but never attended; n = 8 (21.6 %), patient disengaged with neurology service; n = 1 (2.7 %), diagnosed with dementia prior to availability of intervention; n = 5 (13.5 %), reason unknown; n = 6 (16.2 %).
Since the introduction of this intervention, uptake has been high. Of the patients who had the option of attending the intervention (n = 92), 73 patients (79.4 %) attended. Of those 73 who attended, 31 (42.5 %) were followed up to review progress with previously given brain health advice. Patients not followed up had either already been discharged, lost to follow-up, or seen less than a year since their initial appointment.
6.6. Patient characteristics
The prevalence of cardiovascular Risk Factors in our population are presented in Table 2.
Table 2.
Prevalence of cardiovascular risk factors in brain health clinic population (n = 110).
| Cardiovascular Risk Factor | N (%) | Mean (SD) | Range | Missing Data N (%) |
|---|---|---|---|---|
| Hypertension | 54 (49.1) | 8 (7.3) | ||
| High Cholesterol | 52 (47.3) | 26 (23.6) | ||
| Diabetes Status | 11 (10) | |||
| Diabetic | 17 (15.5) | |||
| Pre-Diabetic | 15 (13.6) | |||
| Smoking Status | 9 (8.2) | |||
| Current Smoker | 10 (9.1) | |||
| Ex-Smoker | 43 (39.1) | |||
| Body Mass Index kg/m2 | 28.0 (5.9) | 18.3 – 45.9 | 55 (50) | |
| Drinks Alcohol | 69 (62.7) | 9 (8.2) | ||
| >14 units per week | 23 (20.9) | |||
| History of Heavy Drinking | 15 (13.6) | |||
| Weekly Consumption (units) | 13.7 (16.0) | 1 – 112 | 6 (5.5) | |
| Exercise Status | 32 (29.1) | |||
| No regular exercise | 18 (16.4) | |||
| >NHS Recommendations* | 37 (33.6) | 51 (46.4) |
Note.* As per NHS weekly recommendations – 150+ minutes of moderate exercise or 75+ minutes of vigorous exercise.
Table 2 demonstrates that a high proportion of patients had cardiovascular risk factors e.g. high blood pressure, high cholesterol, levels, diabetes, pre-diabetic, ex-smokers, overweight body mass index [27], as well as drinking more alcohol [28] and exercising less than NHS recommendations [29].
6.7. Other dementia risk factors
The prevalence of previous head injury was n = 26 (23.6 %). Missing data on head injury; n = 31 (28.2 %). At baseline, the prevalence of affective disorders was as follows; low mood; n = 61 (55.5 %), anxiety; n = 61 (55.5 %), co-morbid low mood and anxiety; n = 44 (40 %). Twenty-eight patients (25.4 %) had no diagnosis of anxiety, depression or Functional Cognitive Disorder (FCD).
Sleep quality was sub-optimal in our population. Data on sleep duration was available for 77 patients. Mean hours of sleep per night; 6.41(SD:6.4), range 2.5 – 11.5 h. Prevalence of clinically diagnosed sleep disorders; Obstructive Sleep Apnoea; n = 21 (19.1 %), REM Sleep Behaviour Disorder; n = 4 (3.6 %). Forty-five patients completed the PSQI. The mean score was 7.6 (SD: 3.8), which is classified as ‘poor sleep quality’. Range of scores was 0–15. Twenty-nine (64.4 %) patients who completed the PSQI scored within the poor sleep quality range. The ESS was completed by 48 patients. Mean score; 8.1 (SD 5.2), range 0–18. Fourteen (29.2 %) patients who completed the ESS scored >11, which is possibly suggestive of an underlying sleep disorder.
Hearing loss was also prevalent and was reported in 44 patients (40 %). Data was missing for n = 31 (28.2 %). Of those with hearing loss, n = 20 (45.5 %) used a hearing aid. Forty patients (36.4 %) had attended a hearing check before and n = 21 (19.1 %) attended regular hearing checks. It is unknown whether n = 51 (46.4 %) had ever attended a hearing check before.
6.8. Research participation
In total, sixty-six patients (60 %) had participated in at least one research study at the Brain Centre. Forty-eight patients (43.6 %) consented to the use of their routine clinical data for research purposes. Ten (9.1 %) declined participation, four (3.6 %) indicated interest but consent was not recorded (e.g. Consent form not returned in post (n = 4, 3.6 %), 11 (10 %) missed the opportunity to discuss DREAMS-AD participation due to cancelled appointments/ DNA. DREAMS-AD [22] opened to recruitment in February 2022, therefore some patients seen in the early years of clinic did not get the opportunity to participate, n = 37 (33.6 %).
We do not have the data available regarding the decline rates for other research studies over the years.
6.9. Group comparisons – csf biomarker positive and negative groups
The 38 patients who had CSF biomarker testing for AD were classified as biomarker positive or negative to determine group characteristics. Biomarker results were defined using normal ranges from UCL's Neuroimmunology and CSF Laboratory [30]. Of the patients tested, 17 were biomarker positive (44.7 %) and 21 were biomarker negative (55.3 %). Biomarker positive patients all had an abnormal Amyloid-Beta 1–42/Amyloid-Beta 1–40 Ratio [31]. Biomarker negative patients had values in the normal range for all the CSF biomarkers tested (See Supplementary Information for list of biomarkers). One patient had an isolated, abnormal Amyloid-Beta-42 level, and one patient had abnormal Amyloid-Beta-42 and Total Tau. These two cases were reviewed by a Clinician (E.C.) and not felt to be consistent with a diagnosis of AD. Neither patient declined cognitively or functionally over time. Due to the small sample sizes of these biomarker groups, further statistical analysis to determine significant group differences was not considered appropriate and descriptive statistics were performed instead. These suggested that the biomarker negative group were younger on average and had numerically higher rates of low mood, anxiety, OSA, obesity, excess alcohol consumption, hypertension, diabetes and prediabetes. Sleep duration and quality also appeared to be poorer (See Appendix A).
7. Patient outcomes
7.1. Diagnosis
After appropriate neuropsychological test results and clinical review, a clinical diagnosis was made where possible. Underlying aetiological disorders are provided in Table 3. The underlying cause of cognitive difficulties was unknown for a proportion of patients; n = 44 (40 %) as follows: patient still undergoing diagnostic work-up/investigations; n = 9 (20.9 %), patient declined further investigations; n = 2 (4.7 %), patient disengaged with service whilst under review; n = 6 (14.00 %), cause uncertain but symptoms stable/no clear evidence of neurodegenerative disease; n = 26 (60.5 %).
Table 3.
Disease aetiologies recorded in brain health clinic cohort (N = 110).
| Disease Aetiology | N (%) |
|---|---|
| Uncertain Cause | 44 (40) |
| Alzheimer's Disease | 23 (20.9) |
| Functional Cognitive Disorder | 16 (14.6) |
| Vascular | 8 (7.3) |
| Multi-Factorial | 6 (5.5) |
| Parkinson's Disease | 3 (2.7) |
| Lewy Body Disease | 3 (2.7) |
| Affective Disorders | 3 (2.7) |
| Other | 2 (1.8) |
| Mixed | 2 (1.8) |
Note. ‘Multi-factorial’ included a combination of factors including medical co-morbidities, medication side-effects, sleep disorders, affective disorders, and cardiovascular risk factors.‘Other’ Causes included Alcohol Misuse (n = 1), Radiotherapy (n = 1).
At the time of analysis 29 patients were recorded as MCI (26.4 %), and 22 as SCI (20 %). Aetiology of MCI in these individuals were as follows: Prodromal AD MCI; n = 4 (13.8 %). Vascular MCI; n = 7, (24.1 %), MCI due to Radiotherapy; n = 1 (3.4 %). MCI due to Parkinson's Disease; n = 1, (3.4 %). MCI due to Lewy Body Disease; 1 (3.4 %). MCI due to a multifactorial cause; n = 1 (3.4 %). MCI due to Uncertain Cause, n = 14, (48.3 %), of which n = 7 were AD biomarker negative (24.1 %). At the time of analysis 22 patients were recorded as progressing to dementia. As previously stated, one patient fulfilled criteria for dementia upon entry to the BHC, for a total of n = 23 (20.9 %) patients with a dementia diagnosis.
7.2. CSF biomarker groups – progression vs Non- Progression to dementia
Of the CSF biomarker negative patients (n = 21) one patient (4.8 %) progressed to Dementia (Lewy Body Disease Dementia). Of the 17 Biomarker positive patients. n = 12 (70.6 %) progressed to fulfil criteria for dementia.
7.3. Follow-up and discharge
Seventy patients were discharged (63.6 %). Mean follow-up time in clinic was 3 years (SD:1.4). Follow-up ranged from 4.6 months – 5.9 years. Of the patients who developed dementia (n = 22, 20 %), the average time to receive a diagnosis from first review in clinic was 2.0 years (SD 1.4) but ranged from 3.7 months – 4.7 years.
Of the discharged patients, main reasons for this included: stable or resolved cognitive symptoms; n = 26 (37.1 %), diagnosed with dementia and referred for post-diagnostic support; n = 18 25.7 %) discharged to care of another team; n = 12 (17.1 %), patient disengaged with service or self-discharged, n = 9 (12.9 %), normal test results; n = 3 (4.3 %), patient deceased; n = 1 (1.4 %), reason unclear; n = 1 (1.4 %).
8. Patient experience of the brain health clinic
Over the course of this 6-year study, two questionnaires have been employed (a more simplified version used until 2023) accounting for variations in responses.
8.1. Feedback on service
Of the 19 patients surveyed, all would recommend our service and n = 16 (84.2 %) were extremely likely to. Of 14 surveyed patients, n = 10 (71.4 %) were happy with follow-up frequency, and n = 4 (28.6 %) would have liked more frequent follow-up. Comments generally suggested a high level of satisfaction with medical staff and advice provided.
8.2. Feedback on impact on life and ability
Of 13 surveyed patients, n = 8 (61.5 %) felt better able to cope with symptoms after attending clinic, and n = 4 (30.8 %) felt possibly better able to cope. Of 12 surveyed patients, n = 6 (50 %) felt that the lifestyle intervention would make a difference to their life, and n = 5 (41.7 %) felt it could possibly make a difference.
8.3. Evaluation of clinic in line with recommendations from the European task force for brain health services
We evaluated our clinic in line with the four brain health pillars outlined by the European Task Force for Brain Health Services7:
8.3.1. Risk assessment
In line with Recommendations: We offer specialist diagnostic work-up including serological testing, neuroimaging, neuropsychometry, and CSF biomarker testing for AD.
Outside Current Recommendations: We do not currently use formal risk scores/ scales or offer genetic testing for Apolipoprotein 4 (APOE4) or other common risk genes. In line with European Task Force recommendations, we may wish to consider implementing these in clinic.
8.3.2. Risk communication
In line with Recommendations: Risk profiling is discussed in lifestyle sessions in terms of whether an individual's lifestyle factors increase or lower their risk of dementia.
Outside Current Recommendations: Only qualitative risk descriptors are used e.g. ‘lower’ and ‘higher risk’. Recommended risk communication strategies such as frequencies, percentages and mixed framing [6] are not routinely used.
8.3.3. Personalised intervention
In line with Recommendations: We offer a personalised risk reduction intervention as part of our 1:1 brain health sessions. Patients receive bespoke advice based on their individual level of risk.
Outside Current Recommendations: None
8.3.4. Cognitive enhancement
In line with Recommendations: Patients are encouraged to support cognition by keeping physically, socially and mentally active e.g. exercising, learning a new skill, problem solving tasks, socialising.
Outside Current Recommendations: Formal cognitive enhancement programmes are not currently provided within our service.
8.4. Additional factors
In line with previous European Task Force recommendations [32], we recognize the need to target health inequalities to ensure accessibility of our clinic and research trials to under-represented groups. We are currently working with our PPI Lead to outreach into the Chinese and Somalian Communities in Bristol, and referral pathways are under development to facilitate greater accessibility of our service to these communities.
9. Discussion
We present a six-year overview of the Bristol BHC, a service for patients with mild cognitive symptoms. The Bristol BHC offers a range of high quality, cutting-edge assessments providing accurate diagnosis of cognitive disorders in addition to providing bespoke advice on risk factor modification and research opportunities.
9.1. Patient cohort
The most prevalent cognitive diagnosis in our patient cohort was MCI (n = 29). The cohort featured a higher proportion of men than might be expected given that dementia prevalence is higher in women [33]. Medical and psychiatric co-morbidities were prevalent risk factors for dementia in our population [34]. This strengthens the notion that this is an important population to target with preventative interventions for cognitive impairment [12], as well as recruitment into research trials.
There was a high rate of sleep disturbance in our patient group. Poor sleep quality was found in almost two-thirds of patients who completed the PSQI, and one-third of patients who completed the ESS had abnormal levels of daytime sleepiness. Mean sleep duration was 6.4 h, less than the recommended 7–9 h [35]. Almost one-fifth of patients had Obstructive Sleep Apnoea. Sleep disturbance has been postulated to contribute to neuro-inflammatory processes which increase the risk of developing AD, and a bidirectional relationship between sleep and neurodegeneration has been identified [36,37]. Our findings highlight the importance of continuing to identify and treat this risk factor in this patient cohort.
Affective disorders were also highly prevalent in our clinic population (low mood: 55.5 %, anxiety: 55.5 %). Given their close associations with sleep disturbance [38] this highlights another risk factor for neurodegeneration that could be usefully targeted in this population.
9.2. CSF biomarkers for AD
Of the 38 patients who had CSF biomarker testing for AD, 17 had positive biomarkers and correspondingly had a much higher rate of progression to dementia than the biomarker negative group.
The positive CSF biomarker group was more likely to have underlying neurodegenerative disease (e.g. AD), however, the biomarker negative group was found to have by comparison, more multiple medical and psychiatric co-morbidities. One explanation for this observation would be that the biomarker negative group represented individuals with a multi-factorial aetiology underlying their symptoms. However, a larger group size is required to determine the significance of any of these observations.
CSF biomarker testing for AD was useful in the BHC setting. First, a negative test result was useful in certain clinical situations to exclude a diagnosis of AD. For example, when making a diagnosis of FCD. FCD was the second most common cause for cognitive problems in our cohort and is in line with other Cognitive Disorders Clinics where FCD is a common diagnosis [39]. FCD is also often prevalent in younger patients with more psychiatric and sleep disturbances [40], possibly explaining its relatively high prevalence in our sample. A second benefit of CSF Biomarker testing was to confirm underlying neurodegenerative disease after an informed clinical discussion and guided by patient preference. With the advent of DMTs, it is likely biomarker testing will be offered when milder cognitive symptoms are present. Where patients are biomarker negative, the therapeutic mainstay may be tighter control of cardiovascular risk factors and treatment of underlying mood and sleep problems.
Our dementia progression rates in clinic were lower than in other reported cohorts, where annual progression rates are estimated between 10 and 15 % [41]. However, this likely reflects the heterogeneous nature of our clinic population and varying follow up rates.
As our surveyed group was small, and we have no comparison group, we need to be guarded when drawing conclusions from the patient feedback available. Nevertheless, it was generally positive and in line with that reported for similar brain health services [42]. Uptake for the lifestyle intervention was high at 66 % and suggests that at least a proportion of our patients were keen to engage in a proactive approach to reducing their risk, with nearly two-thirds of surveyed patients feeling better able to manage their symptoms following clinic attendance. As 45.3 % of dementia cases could potentially be prevented by leading a healthy lifestyle [11], promoting a sense of agency in self-management of dementia risk is essential. Making changes to one's own health and lifestyle is a complex behavioral process. This involves motivation, a readiness to change, and often the development and maintenance of healthier coping strategies [43,44]. This presents a challenge where depression or apathy are present. Participants in the FINGER Study with depressive symptoms were less adherent to their exercise programme and experienced less improvement in some aspects of cognition [45]. Affective disorders were common in our population, therefore appropriate management of affective disorders should continue to be a first line priority to facilitate optimum engagement with our lifestyle intervention.
We evaluated our clinic in line with the four brain health pillars outlined by the European Task Force [6]. We met recommendations in most areas. Areas of strength included our risk assessment and personalised intervention pillars, due to the range of investigations and personalised risk reduction intervention on offer. Our cognitive enhancement pillar is an area for future focus. In line with most services in the UK, we do not offer pre-symptomatic genetic testing. However, APOE4 gene homozygosity can lead to the accumulation of CSF biomarkers for AD at an earlier age [46] and therefore it is worth considering how this testing could be incorporated into this clinical setting.
Finally, our evaluation suggests that this clinic provides a gateway into research (60 % participation rate). Research recruitment and participation is an area of priority outlined by the Alzheimer's Society [47] and is key to overcoming the challenges of diagnosing and managing neurodegenerative diseases effectively.
10. Study limitations
-
1)
Variation in clinical assessment over time has led to missing data (e.g. hearing loss not consistently recorded). This may have reduced our ability to identify key relationships between risk factors and cognitive decline.
-
2)
Data on ethnicity was only available from 70.9 % of participants’ medical records, highlighting a potential difficulty with accurately recording this data within NHS systems. Given that the ethnic background of some of our patients was unknown, we are unable to measure health inequalities due to ethnic background. Certain ethnic groups are often under-represented in clinics and research [48,49] and these health inequalities could jeopardize the generalizability of findings.
-
3)
Some of our data on risk factors was qualitative. We acknowledge that certain risk factors (e.g. alcohol consumption) may be under-reported due to stigma [50]. In individuals with MCI, subjective measures of sleep quality do not correlate with objective measures, possibly due to issues with memory recall [51]. Therefore, the subjective nature of some data could potentially affect the validity and accuracy of inferences drawn regarding relationships.
-
4)
Small biomarker group sizes hindered our ability to determine any significant differences in characteristics within groups due to low statistical power, and would require a larger study cohort. This, and the lack of a control group for our lifestyle intervention, require evaluation in the context of a larger study. The small study size also limits the robustness of comparisons made longitudinally, and we would require a larger study to confirm these findings.
-
5)
The lack of a control group for the lifestyle intervention is also a limitation of this study.
-
6)
Despite being an established risk factor for dementia, exposure to environmental risk factors such as air pollution are not currently assessed in our clinic [34]. Other factors such as nutrition and social isolation were discussed in clinic, however data was not presented due to these factors currently being measured in a less objective manner.
11. Future directions
-
1)
There is evidence to suggest that Cognitive Remediation Therapy (which involves targeted training of different cognitive domains), can improve verbal memory, mood and sleep in individuals with MCI [52,53]. Our study would suggest that our population would be keen to engage in this type of intervention and we aim to develop our ‘Cognitive Enhancement’ brain health pillar further [6].
-
2)
Future studies would benefit from an objective outcome measure (assessing effectiveness and accessibility of lifestyle interventions) and standardised pathway for assessment). In addition, we aim to explore the adoption of formal risk scores and genetic testing which may become increasingly available at this time of emerging disease modifying treatments.
-
3)
We aim to consider assessing environmental risk factors such as air pollution in our clinic population, and to explore more objective methods for quantifying social isolation and nutrition as risk factors.
12. Conclusions
The Bristol BHC represents a key population who may be eligible for future disease modifying treatments for Alzheimer's disease. We have demonstrated that there is a high prevalence of risk factors for dementia in this group that may be amenable to intervention. We have also highlighted a cohort of patients with cognitive symptoms secondary to multiple co-morbidities who may benefit from different interventions to those who are biomarker positive for AD. Our data suggest that lifestyle interventions were well received by patients and that many are proactive in altering their lifestyle to reduce dementia risk. Future studies should aim to assess their efficacy.
Funding
This research project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This study was, however, carried out at the National Institute for Health and Care Research (NIHR) Biomedical Research Centre.
Ethical standards
Due to the confidential nature of our data, we present pseudonymised data to protect patient privacy. A large proportion of patients gave written informed consent for clinical data to be used as part of the DREAMS-AD Study protocol [22], which was approved by Frenchay Research Ethics Committee (6/12/2021, REC Reference: 21/SW/0132.) Proportionate ethical approval from the HRA for the PRECISE Study (Predicting Cerebrospinal Fluid Status from Seven Day Memory) (16/07/2023, REC Reference: 23/HRA/2444) also supported the publishing of pseudonymised BHC patient data. Informed consent was not required due to the retrospective nature of this study involving a review of pre-collected clinical data. Our study was conducted in accordance with the relevant guidelines and regulations, including the principles of the Declaration of Helsinki.
Availability of data and materials
The data that support the findings of this study are not openly available due to our research ethics not permitting the sharing of our dataset for reasons of patient confidentiality. Any requests from bona fide researchers for pseudonymised data will be considered by our data access committee and by contacting our primary authors.
Declaration of generative AI and AI-Assisted technologies in the writing process
We confirm that no AI assisted technologies have been used in the writing process.
Open access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/ licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
CRediT authorship contribution statement
Anneka F․ Butters: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Jonathan Blackman: Writing – review & editing, Data curation, Conceptualization. Hannah Farouk: Writing – review & editing, Project administration, Data curation. Saba Meky: Writing – review & editing, Data curation, Conceptualization. Margaret․ A Newson: Writing – review & editing, Data curation, Conceptualization. Tomas Lemke: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Natalie Rosewell: Writing – review & editing, Data curation, Conceptualization. James․ A․ Selwood: Writing – review & editing, Data curation, Conceptualization. Nicholas․ L․ Turner: Writing – review & editing, Methodology. Elizabeth․ J․ Coulthard: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization. Hilary․ A․ Archer: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Elizabeth Coulthard reports a relationship with National Institute for Health and Care Research that includes: funding grants. Elizabeth Coulthard reports a relationship with Rosetrees Trust that includes: funding grants. Elizabeth Coulthard reports a relationship with Bristol Research into Alzheimers and Care of the Elderly that includes: funding grants. Elizabeth Coulthard reports a relationship with Medical research council that includes: funding grants. Elizabeth Coulthard reports a relationship with Biomedical research centre that includes: funding grants. Elizabeth Coulthard reports a relationship with Eisai Inc that includes: consulting or advisory, speaking and lecture fees, and travel reimbursement. Elizabeth Coulthard reports a relationship with Lilly that includes: consulting or advisory, speaking and lecture fees, and travel reimbursement. Elizabeth Coulthard reports a relationship with Biogen Inc that includes: consulting or advisory. Elizabeth Coulthard reports a relationship with Top Hat Clinical Trial (DSMB) that includes: board membership. Elizabeth Coulthard reports a relationship with DESPIAD that includes: board membership. Jonathan Blackman reports a relationship with Alzheimers Research UK that includes: funding grants. Jonathan Blackman reports a relationship with David Telling Charitable Trust that includes: funding grants. Nicholas Turner reports a relationship with National Institute for Health and Care Research that includes: board membership and funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to give special thanks to all patients, staff and researchers who have been involved in the Bristol Brain Health Clinic. We would also like to acknowledge Dr Elizabeth Mallam, Dr Harriet Ball and Dr Aida Moses for their clinical roles in the Cognitive Disorders Clinic.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tjpad.2024.100051.
Contributor Information
Anneka F․ Butters, Email: anneka.butters@bristol.ac.uk.
Hilary․ A․ Archer, Email: hilary.archer@nbt.nhs.uk.
Appendix. Supplementary materials
References
- 1.Alzheimer's Research UK. Dementia is the UK’s biggest killer – we need political action to save lives. 2024. https://www.alzheimersresearchuk.org/blog/dementia-is-the-uks-biggest-killer-we-need-political-action-to-save-lives/#:~:text=Age%20is%20the%20biggest%20risk,than%201.6%20million%20by%202050. Accessed 31 May 2024.
- 2.Alzheimer’s Research UK. What is Alzheimer’s Disease? 2024. https://www.alzheimersresearchuk.org/dementia-information/types-of-dementia/alzheimers-disease/. Accessed 07 November 2024.
- 3.Jessen F., Amariglio R.E., Buckley R.F., et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271–278. doi: 10.1016/s1474-4422(19)30368-0. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen R.C., Doody R., Kurz A., et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. Dec. [DOI] [PubMed] [Google Scholar]
- 5.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Kokmen E., Tangelos E.G. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9(Suppl 1):65. doi: 10.1017/s1041610297004717. -9. [DOI] [PubMed] [Google Scholar]
- 6.Frisoni G.B., Altomare D., Ribaldi F., et al. Dementia prevention in memory clinics: recommendations from the European task force for brain health services. Lancet Reg Health Eur. 2023;26 doi: 10.1016/j.lanepe.2022.100576. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altomare D., Molinuevo J.L., Ritchie C., et al. Brain Health Services: organization, structure, and challenges for implementation. A user manual for Brain Health Services—Part 1 of 6. Alzheimers Res Ther. 2021;13(1):168. doi: 10.1186/s13195-021-00827-2. 2021/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell N.L., Unverzagt F., LaMantia M.A., Khan B.A., Boustani M.A. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. 2013;29(4):873–893. doi: 10.1016/j.cger.2013.07.009. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopman D.S., Petersen R.C. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10):1452–1459. doi: 10.1016/j.mayocp.2014.06.019. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrner J., Kogler S., Lamm C., et al. Awareness of memory deficits in subjective cognitive decline, mild cognitive impairment, Alzheimer's disease and Parkinson's disease. Int Psychogeriatr. 2015;27(3):357–366. doi: 10.1017/S1041610214002245. [DOI] [PubMed] [Google Scholar]
- 11.Livingston G., Huntley J., Liu K.Y., et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet North Am Ed. 2024;404(10452):572–628. doi: 10.1016/S0140-6736(24)01296-0. [DOI] [PubMed] [Google Scholar]
- 12.Kivipelto M., Solomon A., Ahtiluoto S., et al. The finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657–665. doi: 10.1016/j.jalz.2012.09.012. Nov. [DOI] [PubMed] [Google Scholar]
- 13.Budd Haeberlein S., Aisen P.S., Barkhof F., et al. Two randomized phase 3 studies of aducanumab in early alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197–210. doi: 10.14283/jpad.2022.30. 2022/04/01. [DOI] [PubMed] [Google Scholar]
- 14.McDade E., Cummings J.L., Dhadda S., et al. Lecanemab in patients with early Alzheimer's disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi: 10.1186/s13195-022-01124-2. Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois B., von Arnim C.A.F., Burnie N., Bozeat S., Cummings J. Biomarkers in Alzheimer's disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res Ther. 2023;15(1):175. doi: 10.1186/s13195-023-01314-6. 2023/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liss J.L., Seleri Assunção S., Cummings J., et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer's disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med. 2021;290(2):310–334. doi: 10.1111/joim.13244. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julayanont P., Tangwongchai S., Hemrungrojn S., et al. The Montreal Cognitive Assessment-Basic: a Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults. J Am Geriatr Soc. 2015;63(12):2550–2554. doi: 10.1111/jgs.13820. Dec. [DOI] [PubMed] [Google Scholar]
- 18.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. May. [DOI] [PubMed] [Google Scholar]
- 19.Johns M.W. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Kaufer D.I., Cummings J.L., Ketchel P., et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. Spring. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 21.Sikkes S.A., Knol D.L., Pijnenburg Y.A., de Lange-de Klerk E.S., Uitdehaag B.M., Scheltens P. Validation of the amsterdam IADL Questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology. 2013;41(1):35–41. doi: 10.1159/000346277. [DOI] [PubMed] [Google Scholar]
- 22.University of Bristol. Determining Rate of Early Memory and Sleep Change in Alzheimer's Dementia. 2022. https://research-information.bris.ac.uk/en/projects/determining-rate-of-early-memory-and-sleep-change-in-alzheimers-d. Accessed 31 May 2024.
- 23.Mlinac M.E., Feng M.C. Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol. 2016;31(6):506–516. doi: 10.1093/arclin/acw049. Sep. [DOI] [PubMed] [Google Scholar]
- 24.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. Apr. [DOI] [PubMed] [Google Scholar]
- 26.Islam N., Hashem R., Gad M., et al. Accuracy of the Montreal Cognitive Assessment tool for detecting mild cognitive impairment: a systematic review and meta-analysis. Alzheimers Dement. 2023;19(7):3235–3243. doi: 10.1002/alz.13040. Jul. [DOI] [PubMed] [Google Scholar]
- 27.NHS. Obesity. 2023. https://www.nhs.uk/conditions/obesity/. Accessed 31 May 2024.
- 28.NHS. Alcohol Units. 2024. https://www.nhs.uk/live-well/alcohol-advice/calculating-alcohol-units. Accessed 31 May 2024.
- 29.NHS. Physical Activity Guidelines for Older Adults. 2024 https://www.nhs.uk/live-well/exercise/physical-activity-guidelines-older-adults/. Accessed 31 May 2024.
- 30.University College London Hospitals Trust. Neuroinflammatory and CSF Laboratory. 2023. https://www.uclh.nhs.uk/application/files/8316/9624/1844/NICL_MNG_User_Handbook__v16.13.pdf. Accessed 27 June 2024.
- 31.Delaby C., Estellés T., Zhu N., et al. The Aβ1–42/Aβ1–40 ratio in CSF is more strongly associated to tau markers and clinical progression than Aβ1–42 alone. Alzheimers Res Ther. 2022;14(1):20. doi: 10.1186/s13195-022-00967-z. 2022/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne R., Altomare D., Ribaldi F., Molinuevo J.L., Frisoni G.B., Brayne C. Societal and equity challenges for Brain Health Services. A user manual for Brain Health Services-part 6 of 6. Alzheimers Res Ther. 2021;13(1):173. doi: 10.1186/s13195-021-00885-6. Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beam C.R., Kaneshiro C., Jang J.Y., Reynolds C.A., Pedersen N.L., Gatz M. Differences between women and men in incidence rates of dementia and alzheimer's disease. J Alzheimers Dis. 2018;64(4):1077–1083. doi: 10.3233/jad-180141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livingston G., Huntley J., Liu K.Y., et al. Dementia prevention, intervention, and care: 2024 report of the Lancetstanding Commission. Lancet North Am Ed. 2024;404(10452):572–628. doi: 10.1016/S0140-6736(24)01296-0. [DOI] [PubMed] [Google Scholar]
- 35.Hirshkowitz M., Whiton K., Albert S.M., et al. National Sleep Foundation 2019′s sleep time duration recommendations: methodology and results summary. Sleep Health: J National Sleep Foundation. 2015;1(1):40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Chen J., Peng G., Sun B. Alzheimer's disease and sleep disorders: a bidirectional relationship. Neuroscience. 2024;557:12–23. doi: 10.1016/j.neuroscience.2024.08.008. Oct 4. [DOI] [PubMed] [Google Scholar]
- 37.Ju Y.E., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hombali A., Seow E., Yuan Q., et al. Prevalence and correlates of sleep disorder symptoms in psychiatric disorders. Psychiatry Res. 2019;279:116–122. doi: 10.1016/j.psychres.2018.07.009. Sep. [DOI] [PubMed] [Google Scholar]
- 39.Cabreira V., McWhirter L., Carson A. Functional cognitive disorder: diagnosis, treatment, and differentiation from secondary causes of cognitive difficulties. Neurol Clin. 2023;41(4):619–633. doi: 10.1016/j.ncl.2023.02.004. Nov. [DOI] [PubMed] [Google Scholar]
- 40.Cabreira V., Frostholm L., McWhirter L., Stone J., Carson A. Clinical signs in functional cognitive disorders: a systematic review and diagnostic meta-analysis. J Psychosom Res. 2023;173 doi: 10.1016/j.jpsychores.2023.111447. Oct. [DOI] [PubMed] [Google Scholar]
- 41.Farias S.T., Mungas D., Reed B.R., Harvey D., DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oxford Brain Health Clinic. 2024. https://oxfordhealthbrc.nihr.ac.uk/our-work/brain-health-centre/. Accessed 31 May 2024.
- 43.Livia B., Elisa R., Claudia R., et al. Stage of change and motivation to a healthier lifestyle before and after an intensive lifestyle intervention. J Obes. 2016;2016 doi: 10.1155/2016/6421265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espinosa-Salas S., Gonzalez-Arias M. Behavior Modification for Lifestyle Improvement. StatPearls. 2024 [PubMed] [Google Scholar]
- 45.Neuvonen E., Lehtisalo J., Ngandu T., et al. Associations of depressive symptoms and cognition in the finger trial: a secondary analysis of a randomised clinical trial. J Clin Med. 2022;11(5) doi: 10.3390/jcm11051449. Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortea J., Pegueroles J., Alcolea D., et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer's disease. Nat Med. 2024;30(5):1284–1291. doi: 10.1038/s41591-024-02931-w. 2024/05/01. [DOI] [PubMed] [Google Scholar]
- 47.Alzheimer's Society. Our Research Mission Statement. 2022. https://www.alzheimers.org.uk/sites/default/files/2022-06/Alzheimers-Society-Research-Mission-Statement-2022-2027.pdf. Accessed 31 May 2024.
- 48.Bibbins-Domingo K., Helman A, editors. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. National Academies Press; Washington, D.C: 2022. [PubMed] [Google Scholar]
- 49.UK Health Security Agency. Health Matters: health Inequalities and Dementia. 2016. https://ukhsa.blog.gov.uk/2016/03/22/health-matters-health-inequalities-and-dementia/. Accessed 31 May 2024.
- 50.Schomerus G., Lucht M., Holzinger A., Matschinger H., Carta M.G., Angermeyer M.C. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2010;46(2):105–112. doi: 10.1093/alcalc/agq089. [DOI] [PubMed] [Google Scholar]
- 51.DiNapoli E.A., Gebara M.A., Kho T., et al. Subjective-objective sleep discrepancy in older adults with mci and subsyndromal depression. J Geriatr Psychiatry Neurol. 2017;30(6):316–323. doi: 10.1177/0891988717731827. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma I., Srivastava D.J., Kumar A., Sharma R. Cognitive remediation therapy for older adults. J Geriatric Mental Health. 2016;3:57. doi: 10.4103/2348-9995.181919. 01/01. [DOI] [Google Scholar]
- 53.Diamond K., Mowszowski L., Cockayne N., et al. Randomized controlled trial of a healthy brain ageing cognitive training program: effects on memory, mood, and sleep. J Alzheimers Dis. 2015;44(4):1181–1191. doi: 10.3233/JAD-142061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to our research ethics not permitting the sharing of our dataset for reasons of patient confidentiality. Any requests from bona fide researchers for pseudonymised data will be considered by our data access committee and by contacting our primary authors.