Abstract
1. Proteoglycan aggregates from bovine nasal cartilage were studied by using electron microscopy of proteoglycan/cytochrome c monolayers. 2. The aggregates contained a variably long central filament of hyaluronic acid with an average length of 1037nm. The proteoglycan monomers attached to the hyaluronic acid appeared as side chain filaments varying in length (averaging 249nm). They were distributed along the central filament at an average distance of about 36nm. 3. Chondroitin sulphate side chains were removed from the proteoglycan monomers of the aggregates by partial chondroitinase digestion. The molecules obtained had the same general appearance as intact aggregates. 4. Proteoglycan aggregates were treated with trypsin and the largest fragment, which contains the hyaluronic acid, link protein and hyaluronic acid-binding region, was recovered and studied with electron microscopy. Filaments that lacked the side chain extensions and had the same length as the central filament in the intact aggregate were observed. 5. Hyaluronic acid isolated after papain digestion of cartilage extracts gave filaments with similar length and size distribution as observed for the central filament both in the intact aggregate and in the trypsin digests. 6. Umbilical-cord hyaluronic acid was also studied and gave electron micrographs similar to those described for hyaluronic acid from cartilage. However, the length of the filament was somewhat shorter. 7. The electron micrographs of both intact and selectively degraded proteoglycans corroborate the current model of cartilage proteoglycan structure.
Full text
PDF


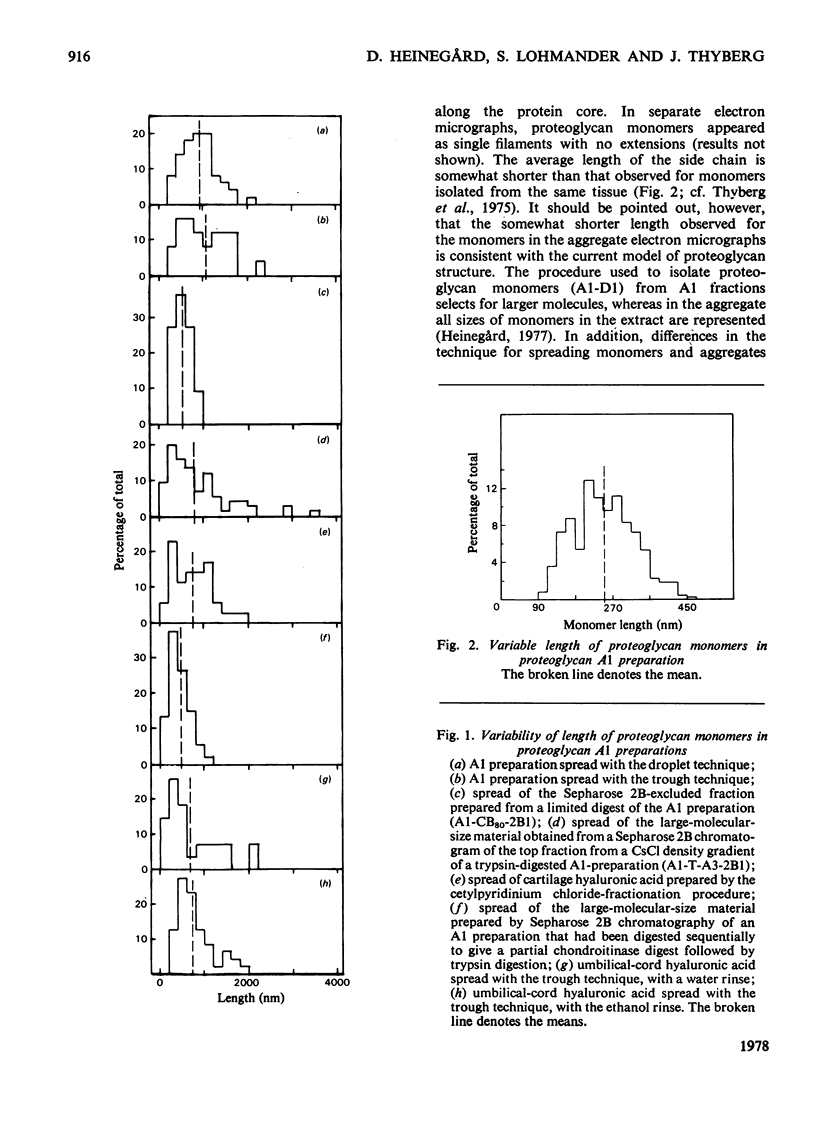
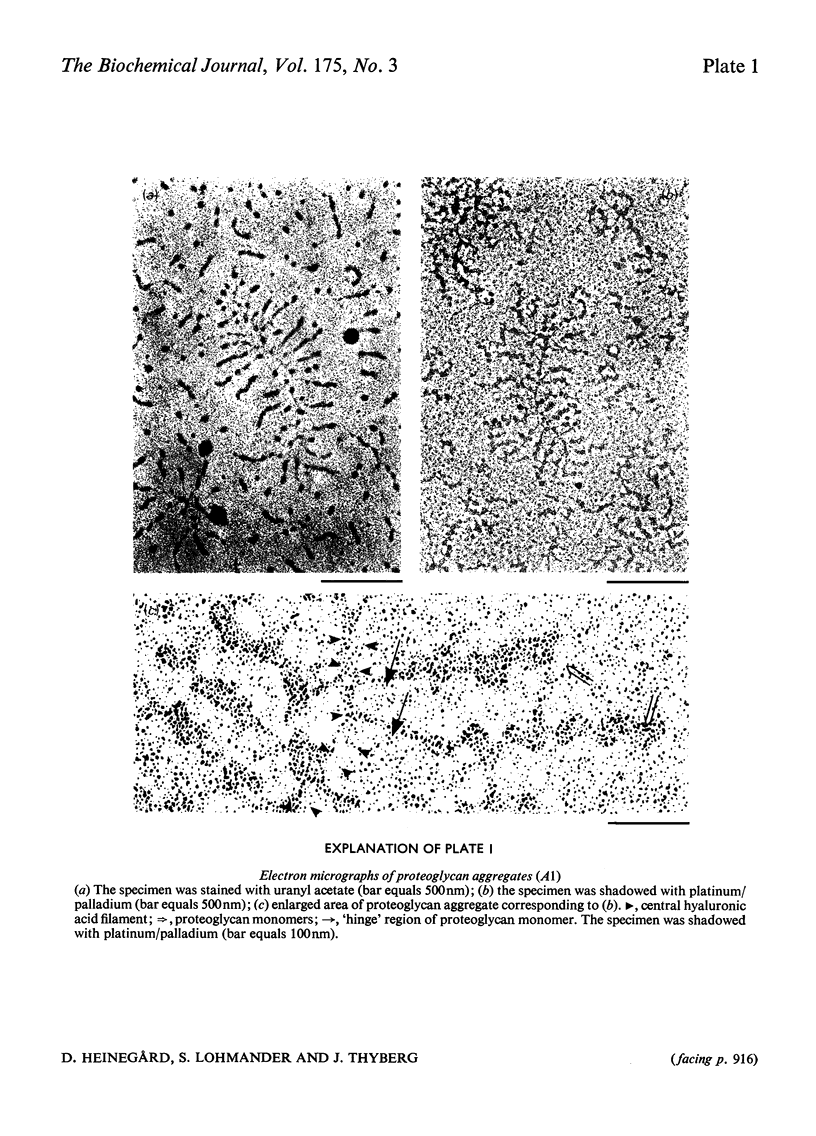
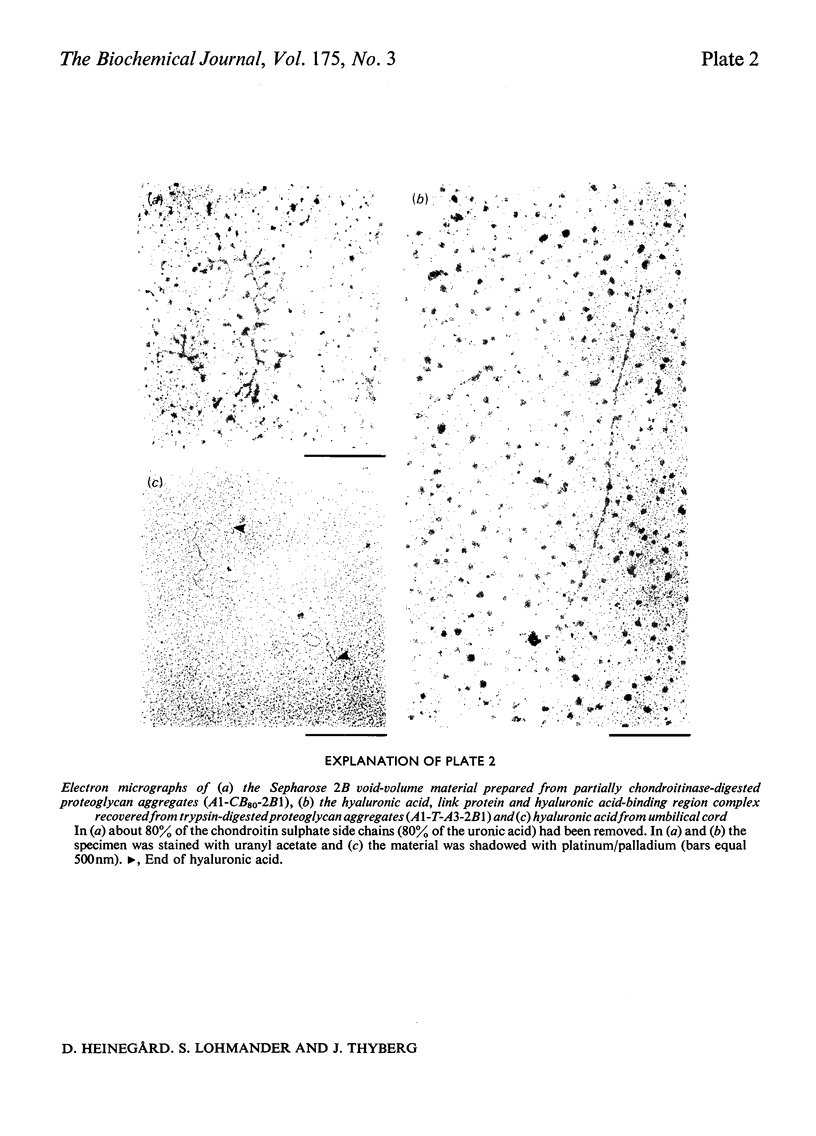



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., BORELIUS E., GARDELL S., HAMNSTROM B., SCOTT J. E. The precipitation of polyanions by long-chain aliphatic ammonium compounds. IV. Elution in salt solutions of mucopolysaccharide-quaternary ammonium complexes adsorbed on a support. Biochim Biophys Acta. 1961 Dec 9;54:213–226. doi: 10.1016/0006-3002(61)90360-2. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Sherblom A. P. Isolation and physical characterization of hyaluronic acid prepared from bovine nasal septum by cetylpyridinium chloride precipitation. J Biol Chem. 1977 Jan 25;252(2):420–426. [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Electron microscopic visualization of the polysaccharide hyaluronic acid. Proc Natl Acad Sci U S A. 1966 Jul;56(1):141–147. doi: 10.1073/pnas.56.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Hyaluronidase digestion and alkaline treatment of bovine tracheal cartilage proteoglycans. Isolation and characterisation of different keratan sulfate proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):193–207. doi: 10.1016/0005-2795(72)90191-2. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- LAURENT T. C., SCOTT J. E. MOLECULAR WEIGHT FRACTIONATION OF POLYANIONS BY CETYLPYRIDINIUM CHLORIDE IN SALT SOLUTIONS. Nature. 1964 May 16;202:661–662. doi: 10.1038/202661a0. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Lohmander S., Heinegård D. Proteoglycans of hyaline cartilage: Electron-microscopic studies on isolated molecules. Biochem J. 1975 Oct;151(1):157–166. doi: 10.1042/bj1510157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P., Wyler T., Buddecke E. Electron microscopic and physico-chemical studies on bovine nasal cartilage proteoglycan. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1043–1052. doi: 10.1515/bchm2.1972.353.2.1043. [DOI] [PubMed] [Google Scholar]