Abstract
The e.p.r. spectra of the Fe-proteins of nitrogenase from all sources studied have unusual features in that they have very anisotropic linewidths and low integrated intensities. These characteristics can be explained by assuming that one of the two electrons accepted by these proteins is located at a rapidly relaxing paramagnetic centre that is unobservable by e.p.r., but causes anisotropic broadening of the e.p.r. signal of the other electron. Complex-formation between Fe-proteins and MgATP is described in terms of a 50-60 degrees rotation of the e.p.r.-observable centre.
Full text
PDF
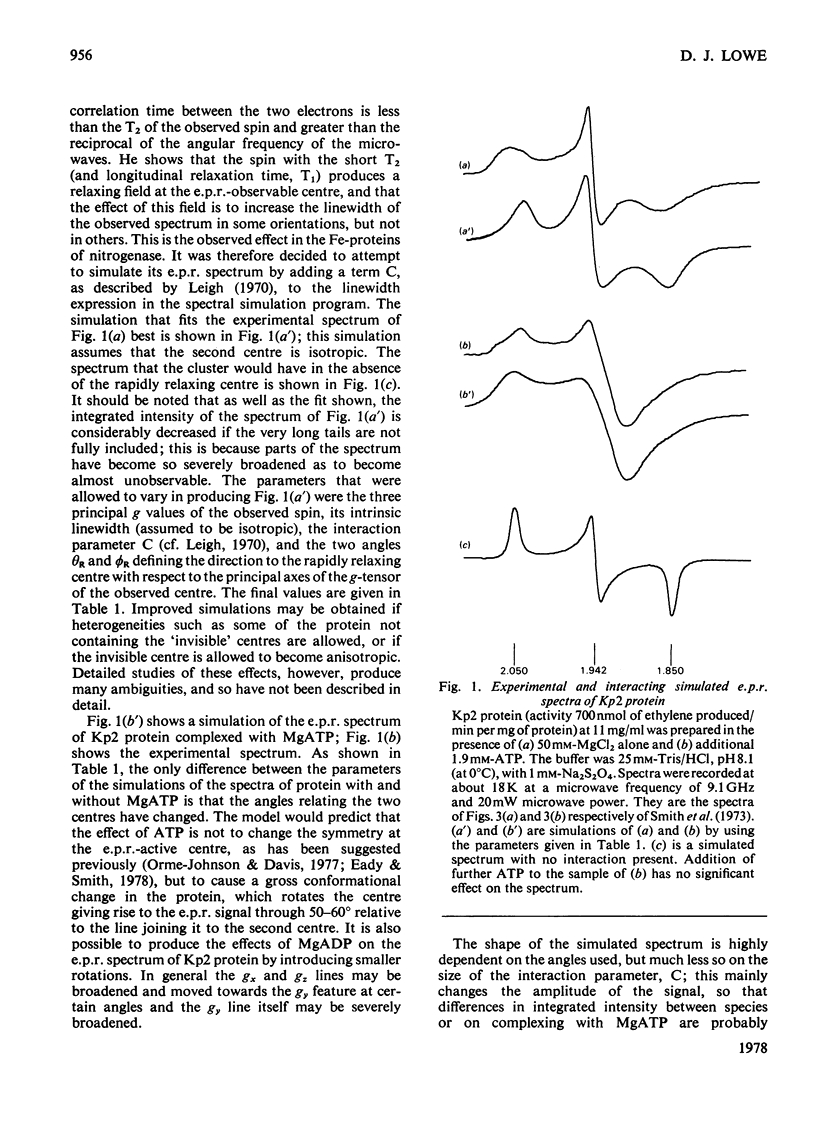
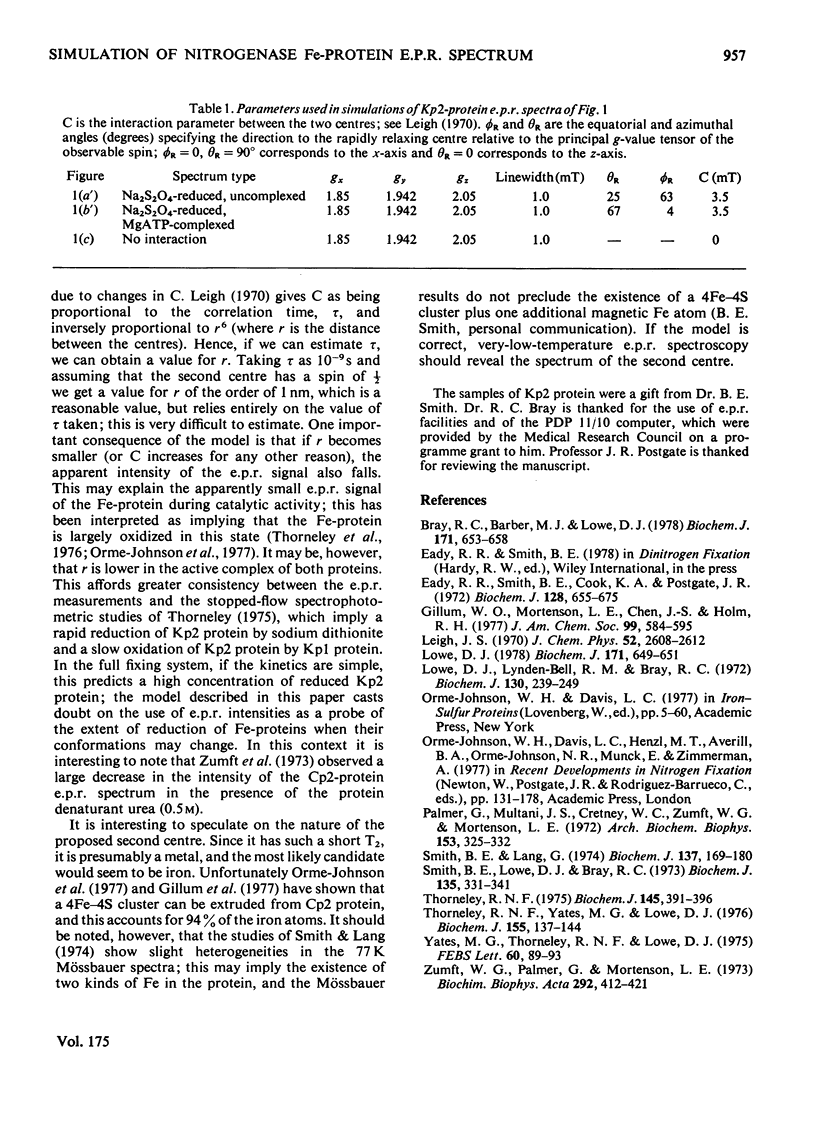
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum W. O., Mortenson L. E., Chen J. S., Holm R. H. Quantitative extrusions of the Fe4S4 cores of the active sites of ferredoxins and the hydrogenase of Clostridium pasteurianum. J Am Chem Soc. 1977 Jan 19;99(2):584–595. doi: 10.1021/ja00444a044. [DOI] [PubMed] [Google Scholar]
- Lowe D. J. Electron paramagnetic resonance in biochemistry. Computer simulation of spectra from frozen aqueous samples. Biochem J. 1978 Jun 1;171(3):649–651. doi: 10.1042/bj1710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G., Multani J. S., Cretney W. C., Zumft W. G., Mortenson L. E. Electron paramagnetic resonance studies on nitrogenase. I. The properties of molybdoferredoxin and azoferredoxin. Arch Biochem Biophys. 1972 Nov;153(1):325–332. doi: 10.1016/0003-9861(72)90452-3. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Lang G. Mössbauer spectroscopy of the nitrogenase proteins from Klebsiella pneumoniae. Structural assignments and mechanistic conclusions. Biochem J. 1974 Feb;137(2):169–180. doi: 10.1042/bj1370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Studies by electron paramagnetic resonance on the catalytic mechanism of nitrogenase of Klebsiella pneumoniae. Biochem J. 1973 Oct;135(2):331–341. doi: 10.1042/bj1350331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N. Nitrogenase of Klebsiella pneumoniae. A stopped-flow study of magnesium-adenosine triphosphate-induce electron transfer between the compeonent proteins. Biochem J. 1975 Feb;145(2):391–396. doi: 10.1042/bj1450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Yates M. G., Lowe D. J. Nitrogenase of Azotobacter chroococcum. Kinetics of the reduction of oxidized iron-protein by sodium dithionite. Biochem J. 1976 Apr 1;155(1):137–144. doi: 10.1042/bj1550137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. G., Thorneley R. N., Lowe D. J. Nitrogenase of Azotobacter chroococcum: inhibition of ADP of the reduction of oxidised Fe protein by sodium dithionite. FEBS Lett. 1975 Dec 1;60(1):89–93. doi: 10.1016/0014-5793(75)80425-x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Palmer G., Mortenson L. E. Electron paramagnetic resonance studies on nitrogenase. II. Interaction of adenosine 5'-triphosphate with azoferredoxin. Biochim Biophys Acta. 1973 Feb 22;292(2):413–421. doi: 10.1016/0005-2728(73)90047-9. [DOI] [PubMed] [Google Scholar]


