Abstract
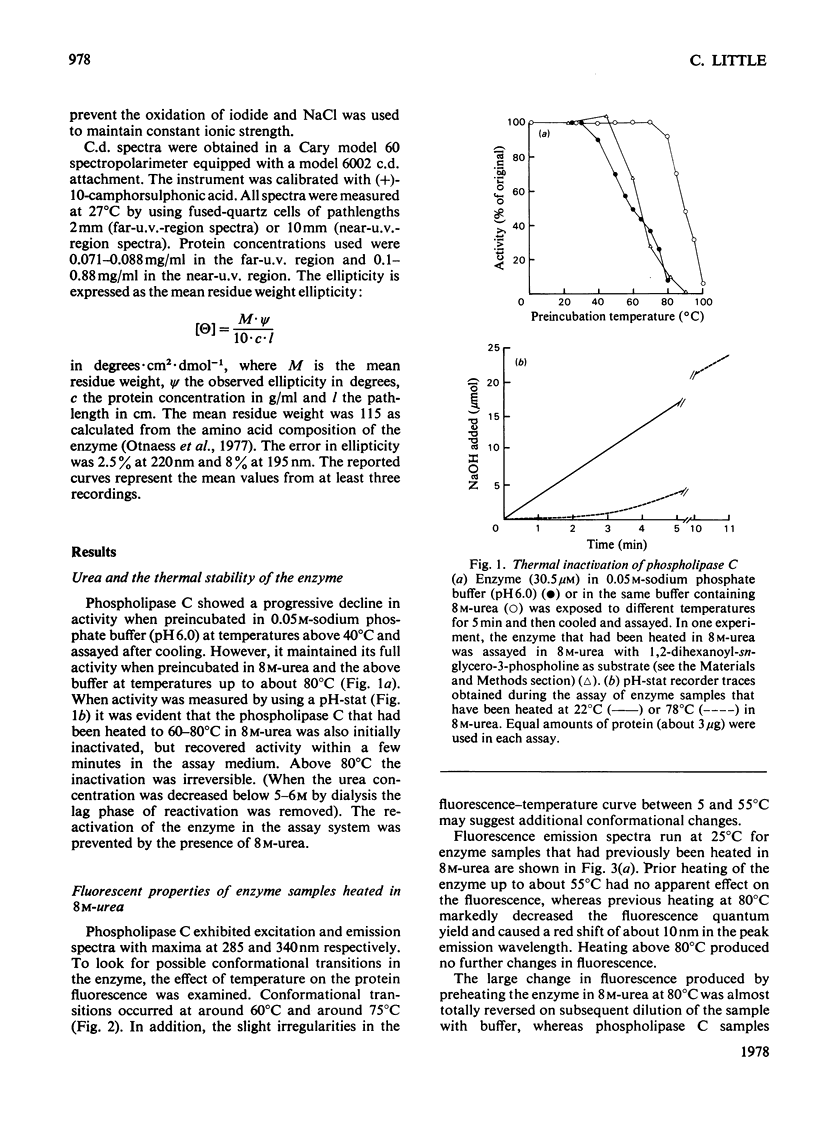
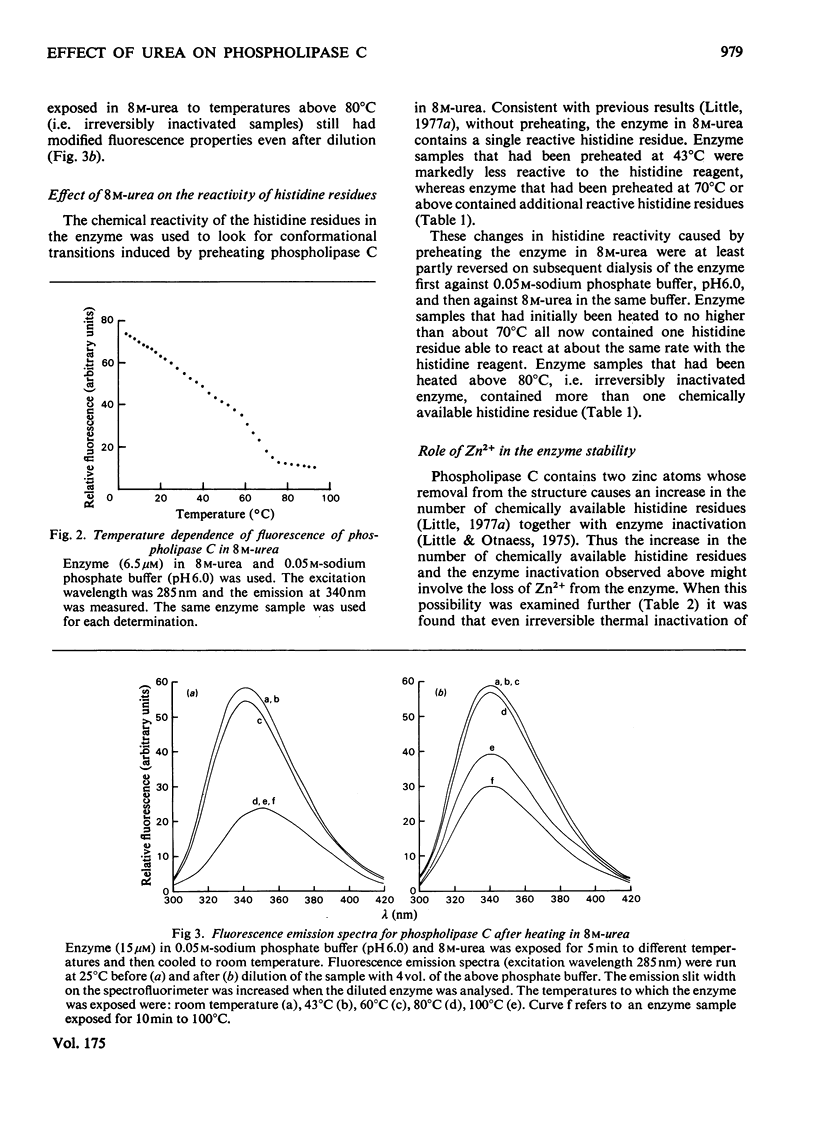
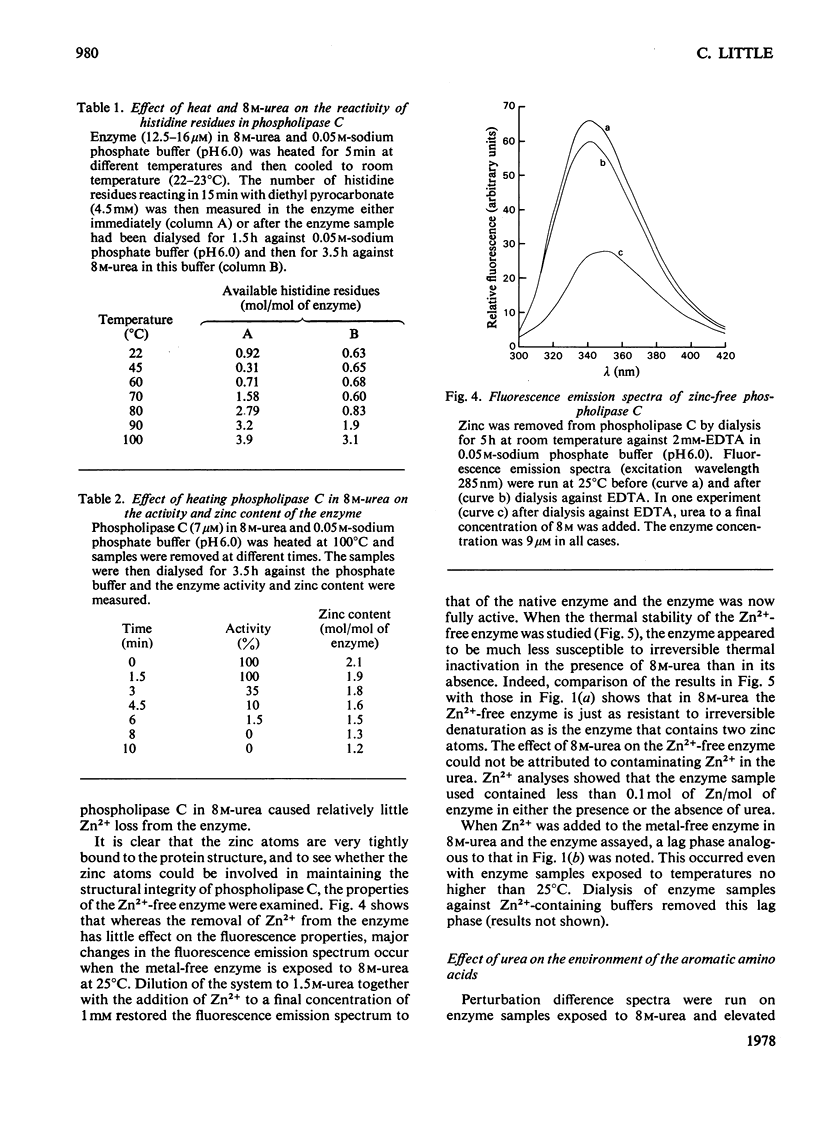
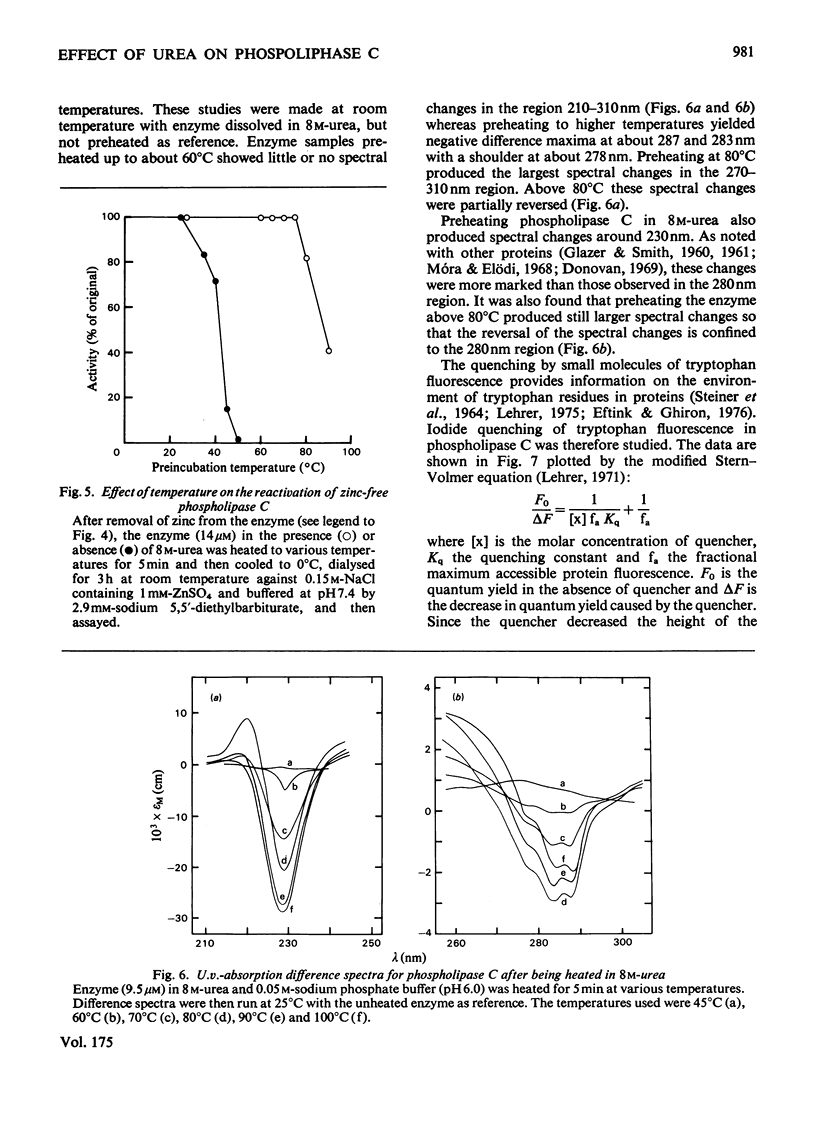
1. When heated in 8 M-urea, phospholipase C(EC 3.1.4.3) from Bacillus cereus undergoes conformational transitions depending on the temperatures used. These transitions were studied by examining protein fluorescence, iodide quenching of protein fluorescence, u.v. difference spectroscopy, chemical availability of histidine residues in the enzyme, circular dichroism and catalytic activity. 2. Unless simultaneously exposed to elevated temperatures the enzyme appears to be unaffected by 8 M-urea. Removal of the two zinc atoms from the enzyme renders phospholipase C very sensitive to denaturation by 8 M-urea as indicated by fluorescence emission spectra and circular dichroism. 3. Both the native and the zinc-free enzymes are markedly more resistant to irreversible thermal inactivation in the presence of 8 M-urea than in its absence. 4. The response of the enzyme to 8 M-urea and the role of zinc in stabilizing the enzyme are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer H. E., Doty P. The conformational stability of alpha-helical nonpolar polypeptides in solution. Biochemistry. 1966 May;5(5):1716–1725. doi: 10.1021/bi00869a038. [DOI] [PubMed] [Google Scholar]
- Beychok S., Armstrong J. M., Lindblow C., Edsall J. T. Optil rotatory dispersion and circular dichroism of human carbonic anhydrases B and C. J Biol Chem. 1966 Nov 10;241(21):5150–5160. [PubMed] [Google Scholar]
- Beychok S. Circular dichroism of biological macromolecules. Science. 1966 Dec 9;154(3754):1288–1299. doi: 10.1126/science.154.3754.1288. [DOI] [PubMed] [Google Scholar]
- Cubero Robles E., van den Berg D. Synthesis of lecithins by acylation of O-(sn-glycero-3-phosphoryl) choline with fatty acid anhydrides. Biochim Biophys Acta. 1969 Dec 17;187(4):520–526. doi: 10.1016/0005-2760(69)90049-6. [DOI] [PubMed] [Google Scholar]
- Doddgh, Radda G. K. 1-Anilinonaphthalene-8-sulphonate, a fluorescent conformational probe for glutamate dehydrogenase. Biochem J. 1969 Sep;114(2):407–417. doi: 10.1042/bj1140407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftink M. R., Ghiron C. A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976 Feb 10;15(3):672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., SMITH E. L. Studies on the ultraviolet difference spectra of proteins and polypeptides. J Biol Chem. 1961 Nov;236:2942–2947. [PubMed] [Google Scholar]
- Hagel P., Gerding J. J., Fieggen W., Bloemendal H. Cyanate formation in solutions of urea. I. Calculation of cyanate concentrations at different temperature and pH. Biochim Biophys Acta. 1971 Sep 28;243(3):366–373. doi: 10.1016/0005-2795(71)90003-1. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Strickland E. H., Billups C. Analysis of the vibrational structure in the near-ultraviolet circular dichroism and absorption spectra of tyrosine derivatives and ribonuclease-A at 77 degrees K. J Am Chem Soc. 1970 Apr 8;92(7):2119–2129. doi: 10.1021/ja00710a054. [DOI] [PubMed] [Google Scholar]
- Hough E., Little C., Jynge K. Preliminary X-ray crystallographic data on phospholipase C from Bacillus cereus. J Mol Biol. 1978 Jun 5;121(4):567–570. doi: 10.1016/0022-2836(78)90401-1. [DOI] [PubMed] [Google Scholar]
- Jirgensons B., de Haas G. H. Circular dichroism of porcine, bovine, and equine pancreatic phospholipases A2 and their zymogens. Unusual conformations simulating helix content. Biochim Biophys Acta. 1977 Oct 26;494(2):285–292. doi: 10.1016/0005-2795(77)90157-x. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Lands W. E. Purification of a phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1969 Dec 17;187(4):477–485. doi: 10.1016/0005-2760(69)90044-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Little C., Aurebekk B., Otnaess A. B. Purification by affinity chromatography of phospholipase C from Bacillus cereus. FEBS Lett. 1975 Apr 1;52(2):175–179. doi: 10.1016/0014-5793(75)80800-3. [DOI] [PubMed] [Google Scholar]
- Little C., Otnåss A. B. The metal ion dependence of phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1975 Jun 24;391(2):326–333. doi: 10.1016/0005-2744(75)90256-9. [DOI] [PubMed] [Google Scholar]
- Little C. The histidine residues of phospholipase C from Bacillus cereus. Biochem J. 1977 Nov 1;167(2):399–404. doi: 10.1042/bj1670399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Móra S., Elödi P. Investigation of the near and far ultraviolet denaturation difference spectra of dehydrogenases. Eur J Biochem. 1968 Sep 24;5(4):574–582. doi: 10.1111/j.1432-1033.1968.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Otnaess A. B., Little C., Sletten K., Wallin R., Johnsen S., Flengsrud R., Prydz H. Some characteristics of phospholipase C from Bacillus cereus. Eur J Biochem. 1977 Oct 3;79(2):459–468. doi: 10.1111/j.1432-1033.1977.tb11828.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. Circular dichroism of globular proteins. A review of the limits of the CD methods for the circulation of secondary structure. Z Klin Chem Klin Biochem. 1974 Sep;12(9):415–422. [PubMed] [Google Scholar]
- Rosenkranz H., Scholtan W. Eine verbesserte Methode zur Konformationsbestimmung von helicalen Proteinen aus Messungen des Circulardichroismus. Hoppe Seylers Z Physiol Chem. 1971 Jul;352(7):896–904. [PubMed] [Google Scholar]
- STEINER R. F., LIPPOLDT R. E., EDELHOCH H., FRATTALI V. ULTRAVIOLET FLUORESCENCE OF PROTEINS: INFLUENCE OF CONFORMATION AND ENVIRONMENT. Biopolym Symp. 1964;13:355–366. [PubMed] [Google Scholar]
- Scanu A. M., van Deenen L. L., de Haas G. H. Optical rotatory dispersion and circular dichroism of phospholipase A2 and its zymogen from porcine pancreas. Biochim Biophys Acta. 1969 Jul 1;181(2):471–473. doi: 10.1016/0005-2795(69)90282-7. [DOI] [PubMed] [Google Scholar]
- Strickland E. H. Aromatic contributions to circular dichroism spectra of proteins. CRC Crit Rev Biochem. 1974 Jan;2(1):113–175. doi: 10.3109/10409237409105445. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Wood E., Dalgleish D., Bannister W. Bovine erythrocyte cupro-zinc protein. 2. Physicochemical properties and circular dichroism. Eur J Biochem. 1971 Jan;18(2):187–193. doi: 10.1111/j.1432-1033.1971.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Complete purification and some properties of phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1971 Apr 13;233(2):474–479. doi: 10.1016/0005-2736(71)90347-6. [DOI] [PubMed] [Google Scholar]


