Abstract
FMPV-1 is a component of FMPV-3, an investigational cancer-specific vaccine and being developed to activate anti-cancer T cell responses targeting frameshift mutations of MSI-H cancers. FMPV-1 is designed to activate T cell responses against transforming growth factor β receptor 2 (TGFβR2) frameshift mutation. Microsatellite instability high (MSI-H) gastrointestinal cancers frequently harbour TGFβR2 frameshift mutations. This first-in-human, phase 1, single centre, open-label study included 16 healthy male subjects who received FMPV-1 (0.15 mg/injection) plus granulocyte-macrophage colony-stimulating factor (GM-CSF) (0.03 mg/injection) as two separate, co-located, injections on Days 1, 8, 15, 29 and 43. All subjects were followed to Day 365. A FMPV-1-specific delayed type hypersensitivity (DTH) skin reactivity test was performed with FMPV-1 (without GM-CSF) on Days 1, 29 and 43 with assessment after 2 days. All subjects were DTH negative at baseline, 8/16 were positive on Day 31 and 15/16 were positive on Day 45. Furthermore, the FMPV-1/GM-CSF induced frameshift mutant TGFβR2-specific T cells after the short vaccination period, and specific T cells were still detectable after 6 and 12 months indicating induction of frameshift mutant TGFβR2-specific T memory cells. Adverse events were limited to mild injection site reactions with no evidence of related systemic signs or symptoms. No other clinically important changes to vital signs, electrocardiograms, haematological, coagulation or laboratory measures related to treatment were observed. FMPV-1/GM-CSF was well tolerated and generated vaccine-specific T cell immune responses in healthy subjects. These findings support clinical studies in patients with, or at risk of, cancers carrying TGFβR2 frameshift mutations.
Clinical trial identification: ClinicalTrials.gov: NCT05238558. EudraCT: 2020-004363-80.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-025-03969-6.
Keywords: Peptide vaccine, Immunotherapy, GM-CSF, TGFβR2, Immune response, Lynch syndrome
Introduction
Microsatellite instability high (MSI-H) occurs in various cancer types and in over 95% of patients with Lynch syndrome, or hereditary non-polyposis colorectal cancer where tumours may develop at a relatively young age [1].
MSI-H/dMMR tumours characteristically involve frameshift mutations, with > 95% representing single nucleotide deletions [2]. The frameshift mutation creates a shift of codons for protein expression, resulting in a mutant protein sequence (neoantigen) foreign to the immune system of the host [3]. Since these neoantigens are cancer-specific, peptides comprising immunogenic fragments (neoepitopes) could potentially serve as both therapeutic or prophylactic vaccines to induce cancer-specific T-cellular immune responses without the risk of off-target effects causing severe adverse effects [4–6]. A promising option involves developing vaccines that generate T cells targeting tumour-specific antigens [7] as this has shown clinically relevant results in various cancer populations [8–12].
FMPV-3 is an investigational cancer-specific vaccine and being developed to activate anti-cancer T cell responses targeting MSI-H cancers including Lynch syndrome with frameshift-mutated transforming growth factor β receptor 2 (TGFβR2), Asteriod1 homolog (ASTE1) and TAF1 , administered with the adjuvant recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF is reported to be an effective adjuvant for protein and peptide vaccines [13] and was selected as adjuvant for FMPV-1. Furthermore, GM-CSF is a clinically well proven adjuvant for cancer-related neoantigen peptide vaccines in clinical studies [9, 14].
The TGFβR2 component (FMPV-1) of FMPV-3 consists of one synthetic peptide of 24 amino acids (fsp2, KGIMKEKKSLVRLSSCVPVALMSA; Bachem AG Switzerland), comprising potential immunogenic fragments and epitopes [15–17] of the naturally occurring human neoantigen protein expressed by frameshift-mutated TGFβR2 which is present in more than 75% in sporadic MSI colorectal cancers and more than 90% in hereditary colorectal cancer (Lynch syndrome). TGFβR2 is a component of a transmembrane receptor–ligand complex common in epithelial cells [18]. Altered downstream TGFβ signalling contributes to various processes driving tumorigenesis and tumour progression [19]. Inactivating TGFβR2 induces invasive growth by reducing cell–matrix interactions and matrix protein production, while increasing the production of cancer-associated cell–cell adhesion proteins, proinflammatory mediators and mitogen-activated protein kinase (MAPK) signalling [20]. FMPV-1 is expected to induce clonal expansion of mutated TGFβR2-specific T cells which will then target and destroy tumour cells [21].
To determine whether FMPV-1, with the adjuvant GM-CSF, can stimulate an appropriate immune response in immune-competent individuals who would be expected to generate an appropriate immune response, a first-in-human study was conducted to assess the safety of the FMPV-1/GM-CSF vaccination and the FMPV-1-specific immune response in healthy subjects.
Materials and methods
Design and ethics
This single centre, open-label, non-randomised study was conducted in 16 healthy male subjects. All subjects provided informed written consent. The study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The clinical study was approved by the UK Medicines and Healthcare Products Regulatory Agency, and the protocol approved by the London – West London & Gene Therapy Advisory Committee Research Ethics Committee. The study is registered at ClinicalTrials.gov (NCT05238558) with EudraCT allocation of 2020-004363-80.
Subjects
Subjects were healthy males aged 18 to 55 years, body mass index 18.0 to 32.0 kg/m2 without clinically important laboratory abnormalities, Gilbert’s syndrome, positive for hepatitis B surface antigen, hepatitis C virus antibody or human immunodeficiency virus antibody, evidence of SARS-CoV-2 infection, a history of serious adverse reaction or hypersensitivity to the investigational agents or clinically relevant allergy, history of alcohol or drug abuse in the last 2 years, or positive alcohol breath test or drugs of abuse breath. Also excluded was participation in a clinical research study of an investigational drug within the previous 90 days, or within 5 elimination half-lives whichever is longer, previous administration of any immunomodulatory drugs within 90 days, or any vaccine within 28 days of enrolment, donation of blood or plasma or loss of > 400 mL of blood within the previous 3 months, administration of any prescribed or over-the-counter medications within the previous 7 days (except vitamins and paracetamol), subjects with tattoos or scars that would impair the assessment of injection site reaction, any malignancy within the last 3 years or a first-degree relative with a haematological malignancy, any other illness or medical condition deemed by the investigator to preclude participation in the study. Subjects with pregnant or lactating partners were excluded. All subjects with a sexual partner of childbearing potential had to use a condom plus an approved method of highly effective contraception from the time of informed consent until 90 days after the final vaccine administration.
Interventions
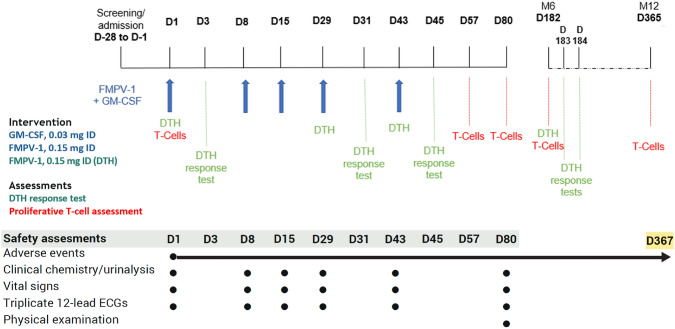
Screening was on Day 28 to Day 2, with admission on Day 1 (Fig. 1). Subjects received GM-CSF (Molgramostim; Amoytop Biotech Co, Ltd, Xiamen, China) 0.03 mg intradermal (ID) followed by FMPV-1 (Baccinex SA, Courroux, Switzerland) 0.15 mg ID administered separately 15 ± 5 min apart to the back of the upper arm in the same injection site on Days 1, 8, 15, 29 and 43. As a precaution, two first subjects formed a sentinel group and were dosed sequentially, 48 h apart, prior to the remaining subjects (main group). The sentinel subjects were observed for safety at the clinical unit for 48 h post-dose. All subjects remained in the clinical unit for at least 3 h following ID injections. An FMPV-1-specific DTH response test was performed on Days 1, 29 and 43 by administration of FMPV-1 0.15 mg ID to the lower area of the contralateral arm 30 ± 5 min before GM-CSF administration. Thus, the total amount of FMPV-1 administered on each DTH Days 1, 29 and 43 was 0.30 mg as these coincided with FMPV-1/GM-CSF vaccination. Follow-up was at Days 80, 182 (6 months) and 365 (12 months) after initiation of dosing.
Fig. 1.
Study scheme
Assessments
Safety
Physical examination was performed at screening and Day 80. Adverse events (AE) were monitored from enrolment until the 12-month follow-up using MedDRA (v24.1). AE severity was graded according to the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (September 2007). The investigator assessed the relationship between AEs and drug administration. Samples were collected for clinical chemistry, haematology, coagulation and urinalysis vital signs, and triplicate electrocardiograms (ECGs) were performed at screening/admission and as per Fig. 1.
Pharmacodynamics
Delayed type hypersensitivity (DTH)
The DTH reaction was assessed on Days 3, 31, 45 and where feasible at Day 182 (7 subjects only). Assessments included the diameter of the reddening and/or induration and the intensity (based on pain, ulceration and induration) of the injection site reaction, with photographs taken of the injected area. DTH response was considered positive if the average skin reaction diameter at the injection site was ≥ 5 mm 48 h post-test. Supplementary materials available on request.
Immunological assessment of FMPV-1-specific T cell response
The presence of FMPV-1 antigen-specific T cells in peripheral blood was assessed on Days 1, 57, 80, 182 and 365. Appropriate procedures were used for isolation of peripheral blood mononuclear cells (PBMCs) and in vitro analyses of T cell response against fsp2 (FMPV-1 peptide).
Frameshift-mutated TGFβR2 cell-free DNA
The presence of frameshift-mutated TGFβR2 cell-free DNA (cfDNA) in plasma samples was assessed by a PCR assay using cfDNA as templates extracted from either the plasma samples obtained on pre-dose on Day 1 or from plasma samples obtained on Day 29.
Outcomes
The safety population included all enrolled subjects receiving at least one dose of FMPV-1/GM-CSF. Primary safety outcomes included evaluation of AE frequency/severity, occurrence of abnormal laboratory results, vital signs, ECGs and physical examination. Secondary outcomes were the frequency of DTH response and reaction intensity.
Statistical analysis
For this exploratory study, no formal sample size calculation was done, and findings were reported using descriptive statistics. Data up to Day 365 were analysed using SAS version 9.4 (SAS Analytics, Cary, NC).
Results
Subjects and intervention
Between 14th January 2022 and 12th October 2022, 16 healthy male subjects were enrolled, and all completed the study to Day 365 per protocol (Fig. 1). Baseline characteristics are shown in Table 1.
Table 1.
Subject baseline characteristics
| Characteristic | FMPV-1 plus GM-CSF (N = 16) |
|---|---|
| Age, years | 39.5 (10.7) [20–53] |
| Sex, male | 16 (100) |
| Ethnicity, race | |
| White | 13 (81.3) |
| Asian | 2 (12.5) |
| Black/African American | 1 (6.3) |
| Height, cm | 177.8 (6.8) [168–197] |
| Weight, kg | 83.74 (10.84) [65.0–103.3] |
| Body mass index, kg/m2 | 26.43 (2.44) [22.0–30.8] |
Data are mean (standard deviation) [range] except for sex and ethnicity, n (%)
Ethnicity and race were self-defined
All subjects received GM-CSF 0.03 mg ID followed by FMPV-1 0.15 mg ID administered separately into the same site according to the protocol (Fig. 1). DTH reaction test for FMPV-1 was performed per protocol out to Day 43 (Fig. 1) with an additional, 7 subjects consented to undergo DTH test at Day 182.
Safety
FMPV-1/GM-CSF was well tolerated with no dose-limiting toxicities, major protocol deviations or serious AEs. Twenty-one treatment-emergent AEs occurred in 11 subjects, most commonly injection site pruritus (6/16; 37.5%) (Table 2). All AEs were Grade 1 in severity, except for two unrelated Grade 3 + AEs resulting from excessive exercise in two subjects. Eight treatment-related AEs occurred in 6 subjects, all Grade 1 pruritus at the injection site (2 at the DTH site to FMPV-1 alone and 6 at the vaccination site to FMPV-1/GM-CSF). There were no allergic or hypersensitivity reactions.
Table 2.
Treatment-emergent adverse events (AE) of any cause
| System class and AE | Number of subjects (%) | Number of events |
|---|---|---|
| Any AE | 11 (68.8) | 21 |
| General disorders and administration site conditions | 7 (43.8) | 11 |
| Chest discomfort# | 1 (6.3) | 1 |
| Injection site pruritus | 6 (37.5) | 8* |
| Fatigue | 1 (6.3) | 1 |
| Pain | 1 (6.3) | 1 |
| Vessel puncture site bruise | 1 (6.3) | 1 |
| Infections and infestations | 2 (12.5) | 2 |
| Asymptomatic COVID-19 | 1 (6.3) | 1 |
| Nasopharyngitis# | 1 (6.3) | 1 |
| Viral upper respiratory tract infection | 1 (6.3) | 1 |
| Musculoskeletal and connective tissue disorders | 2 (12.5) | 2 |
| Exostosis | 1 (6.3) | 1 |
| Pain in extremity | 1 (6.3) | 1 |
| Nervous system disorders | 2 (12.5) | 2 |
| Headache | 2 (12.5) | 2 |
| Sciatica# | 1 (6.3) | 1 |
| Social circumstances | 2 (12.5) | 2 |
| Excessive exercise | 2 (12.5) | 2 |
| Immune system disorders | 1 (6.3) | 1 |
| Seasonal allergy | 1 (6.3) | 1 |
| Respiratory, thoracic and mediastinal disorders | 1 (6.3) | 1 |
| Choking sensation | 1 (6.3) | 1 |
*Related events
#Day 80 to Day 365
Post-baseline abnormal values for haematological, coagulation and clinical chemistry parameters were uncommon and mostly mild (Grade 1). Exceptions included one case of Grade 3 decreased neutrophils (0.95 × 109/L) on Day 43 and one case of Grade 1 increased bilirubin (1.5 × the upper limit of normal [ULN]) on Day 43 both cases resolved by Day 80. One of these cases also experienced Grade 3 increased aspartate aminotransferase (7.8 × ULN; 288 IU/L) and Grade 4 increased creatine kinase (56.5xULN) on Day 80, along with increases in alanine aminotransferase (1.6xULN), lactate dehydrogenase (1.7xULN) and D-Dimer (2.3xULN); these changes were considered clinically relevant but attributed to excessive exercise, reported as an unrelated adverse event (see above). Unscheduled assessments at Day 87 showed normalisation of laboratory results for this subject, though creatine kinase remained mildly elevated (2.5xULN). There were no clinically important changes in vital signs (supplemental material Figure S2). No subject had an increase in Fridericia-corrected QTcF interval > 30 ms from baseline or > 450 ms at any assessment. There were no clinically important changes in other ECG parameters. Between Day 80 and Day 365, no related adverse events were reported.
Immune response
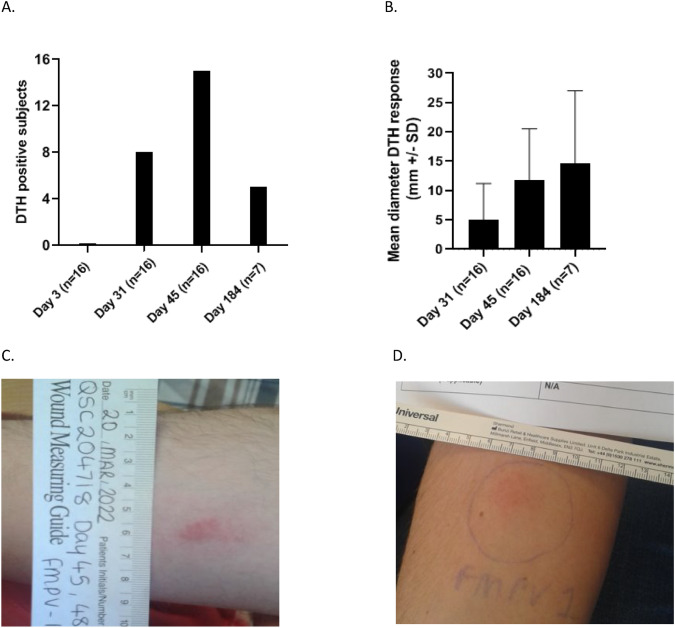
FMPV-1 immunogenicity was assessed by in vivo DTH reactions to ID injections of the FMPV-1 peptide. None of the 16 subjects displayed a positive DTH at Day 3 after the first vaccination, as anticipated. After 3 vaccinations (Day 31) and 4 vaccinations (Day 45), the mean DTH diameter of all 16 subjects (DTH responders and non-responders) was 5 mm (S.D. ± 6.2 mm) and 11.8 (S.D. ± 8.7 mm), and responses were positive for 8/16 (50%) and 15/16 (93.8%) subjects, respectively (Fig. 2). Among the DTH responders, the mean DTH diameter was 10.1 mm (S.D. ± 4.9 mm) after 3 vaccinations (8 responders) and 12.6 mm (S.D. ± 8.4 mm) after 4 vaccinations (15 responders). The non-responsive subject at Day 45 had only 3 fully effective vaccinations up to Day 45 which may potentially explain the lack of positive DTH result at Day 45. At Day 184, the mean diameter of the 7 evaluable subjects (5 responders) was 14.6 mm (S.D. ± 12.4 mm), Fig. 2, Table 3.
Fig. 2.
Delayed type hypersensitivity (DTH) reactions following FMPV-1 challenge. DTH reactions were measured 48 h after ID injections with FMPV-1 peptide only. A DTH positive subjects at Day 3, Day 31, Day 45 (all n = 16) and Day 184 (n = 7). B Mean diameter of DTH reactions (mm ± SD) in evaluable subjects at Day 31 and Day 45 (n = 16) and at Day 184 (n = 7). C. Example of DTH reaction in a subject at Day 45. D Example of DTH reaction in a subject at Day 184 indicating generation of T memory cells
Table 3.
Overview of immune responses by DTH reaction and T cell proliferation analysis after five vaccinations
| Subject | DTH | T cell proliferation | ||||
|---|---|---|---|---|---|---|
| Baseline | Day 31 and/or Day 45 |
Day 184 | Baseline | Day 57 and/or Day 80 | Day 184 and/or Day 365 | |
| 101 | NEG | NEG* | Not tested | NE | NE** | NE** |
| 102 | NEG | POS* | Not tested | NEG | NE** | NE** |
| 103 | NEG | POS | Not tested | NE | NEG | NE |
| 104 | NEG | POS | Not tested | NE | POS | NEG |
| 105 | NEG | POS | Not tested | NE | POS | NEG |
| 106 | NEG | POS | Not tested | NE | NE | NE |
| 107 | NEG | POS | Not tested | NE | NE | NEG |
| 108 | NEG | POS | Not tested | NEG | NE | POS |
| 201 | NEG | POS | POS | POS*** | POS | POS |
| 202 | NEG | POS | POS | NE | NE | NEG |
| 203 | NEG | POS | POS | NEG | NEG | NE |
| 204 | NEG | POS | Not tested | NEG | POS | POS |
| 205 | NEG | POS | NEG | NE | NEG | NEG |
| 206 | NEG | POS | NEG | NE | POS | POS |
| 207 | NEG | POS | POS | NE | POS | POS |
| 208 | NEG | POS | POS | NEG | POS | POS |
DTH reaction was measured 48 h after ID injection with FMPV-1 peptide only
NEG negative DTH reaction, POS positive DTH reaction, NE one or more non-evaluable samples
*Subject 101 and 102 tested after 3 effective FMPV-1/GM-CSF vaccinations, **Subject 101 and 102 received only 4 effective vaccinations, ***SI value 2.01 (borderline positive)
To further characterise the immune response, FMPV-1 antigen-specific T cells in peripheral blood were assessed through in vitro T cell proliferation assays on Days 1, 57, 80, 182 and 365. Stimulation Index (SI) values ≥ 2 were used as a cut-off for positive immune response. Evaluable subjects for T cell analysis post-vaccination were defined as having received all five vaccine doses and T cell count ≥ 5 × 104 / well and not an SI value of SEC 3 stimulated cells < 2. In total 7/10 evaluable subjects showed adequate levels of FMPV-1 antigen-specific T cells in evaluable samples from Day 57 and Day 80 (post-vaccination) and 8/11 evaluable subjects showed FMPV-1 antigen-specific T cells in evaluable samples from Day 182 and/or Day 365 (Table 3). Positive post-vaccination immune responses detected against the FMPV-1 peptide up to Day 80 ranged from SI 2.4 to 21.7 and at Day 182 and Day 365 from 2.1 to 9.6 (Fig. 3A and B).
Fig. 3.
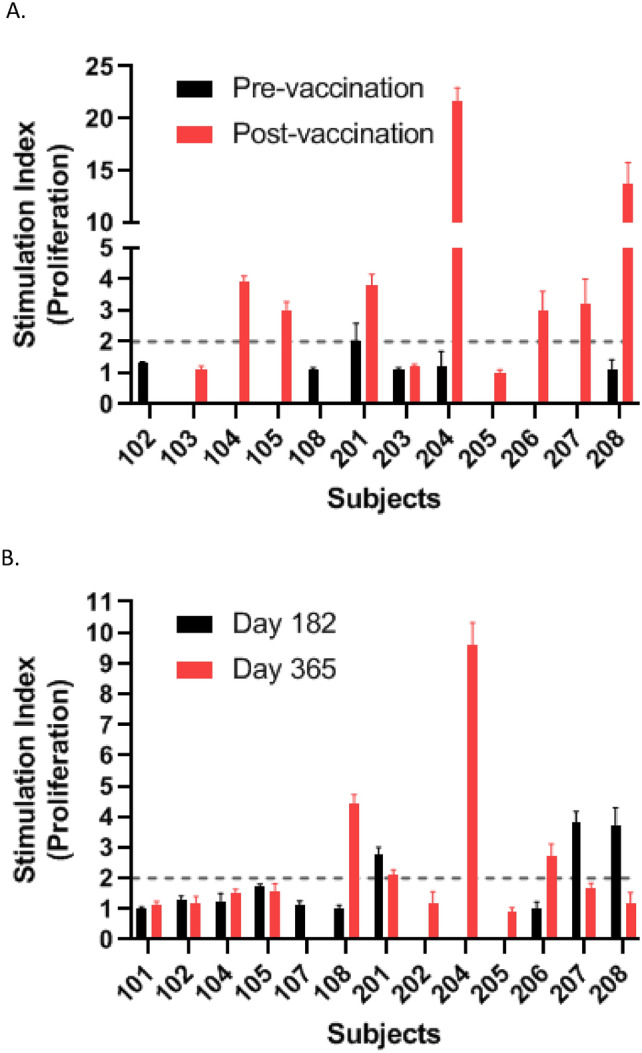
T cell proliferation against FMPV-1 peptide in pre- and post-vaccination blood samples. A The strongest post-vaccination T cell responses detected against the FMPV-1 peptide up to Day 80 for each subject. B Post-vaccination T cell responses against the FMPV-1 peptide at Day 182 and Day 365 for each subject. Stimulation Index (SI) is plotted as mean of triplicates ± SD, SI ≥ 2 was considered immune response. All results with T cell count at Day 12–14 < 5 × 104/well and the SI value of SEC 3 stimulated cells < 2 were excluded from the final analysis
Frameshift-mutated TGFβR2 cell-free DNA
No mutated cfDNA TGFβR2 fragments were detected in plasma taken on Day 1 or Day 29. Given the healthy status of the subjects without cancer history, this absence was expected. Synthetic DNA of both wildtype and mutated TGFβR2 sequences was used as negative and positive controls.
Discussion
In this first-in-human study, the safety and immunogenicity of the FMPV-1 peptide vaccine co-administrated with the adjuvant GM-CSF were evaluated on 5 occasions in 16 healthy male subjects, followed for 12 months. FMPV-1 is an immunogenic peptide fragment of 24 amino acids of the naturally occurring human neoantigen protein expressed by frameshift-mutated TGFβR2, a mutation commonly occurring in MSI-H cancers, which is designed to generate mutant TGFβR2-specific T cells. FMPV-1/GM-CSF was well tolerated with no dose-limiting toxicities or serious adverse events. Only mild injection site adverse reactions were reported, with no evidence of related systemic signs or symptoms. No clinically concerning changes related to FMPV-1/GM-CSF were observed in haematological, coagulation and clinical chemistry values except for changes in laboratory and haematological values in a subject who undertook excessive exercise, which recovered quickly. Due to the short peptide chain, peptide vaccines are less likely to cause allergic or autoimmune responses, and favourable side effect profiles have previously been observed for peptide vaccines combined with GM-CSF [22, 23].
Peptide vaccines, such as FMPV-1/GM-CSF, occupy a unique position to induce effective anti-tumour T cell responses in therapeutic and prophylactic vaccine settings. DTH reactions, also called Type IV hypersensitivity reactions, mainly involve antigen-specific T cell recognition of drug antigens and cell activation [24].
FMPV-1 induced de novo responses specific to the FMPV-1 antigen after a short vaccination period as demonstrated by a higher number of subjects showing an increased mean diameter of DTH reactions after 3 (Day 31) and 4 (Day 45) vaccinations. Additionally, 6/8 subjects with positive DTH reactions showed an intrasubject increase in DTH measurements after both the third and fourth vaccinations. Hence, FMPV-1 demonstrated immunogenicity in non-cancer subjects. Based on these results, we hypothesise that the DTH reaction could serve as a biomarker for in vivo detection of FMPV-1-induced T cell response. Positive DTH reactions persisted in 5 out of 7 subjects approximately 4.5 months post-final vaccination, suggesting induction of mutant TGFβR2-specific memory T cells.
Induction of memory T cells is important not only in therapeutic setting, but also in a prophylactic cancer setting, as they can quickly be reactivated upon encountering cancer cells expressing relevant neoantigens. Both primary and booster vaccinations may impact the effectiveness and duration of antigen-specific T cell responses; hence, individualised vaccination schedules may be required in different cancer subpopulations based on predictive biomarkers for clinical efficacy and monitoring of anti-tumour responses [16].
Studying antigen-specific CD4+ T cells in vitro is technically difficult primarily due to their low frequency in blood. Accurately interpreting true positive and true negative T cell responses is difficult due to the absence of a standardised cut-off value [25, 26]. Stimulation Index (SI) is commonly used to differentiate between immune responders and non-responders, with a cut-off typically between 1.5 and 3 [26–28]. Isolation and preservation procedures for PBMCs are crucial for optimising their quality. Additionally, selecting appropriate cut-off values for monitoring of antigen-specific CD4+ T cell responses avoids misinterpretation of results [26, 29]. In this study, SI ≥ 2 was used as positive response when analysing FMPV-1-specific T cells using the T cell proliferation assay. Owing to impaired quality of PBMC samples and non-optimised T cell proliferation protocols, which were updated during the study to improve yield, viability and FMPV-1-specific T cell responses, several samples were deemed non-evaluable.
7/10 evaluable subjects demonstrated the presence of FMPV-1-specific T cells in blood up to Day 80 after the 5 vaccinations, and 6/11 evaluable subjects showed FMPV-1-specific T cells after 6 and/or 12 months, further supporting the DTH results that the FMPV-1 peptide vaccine is immunogenic. Limited numbers of PBMCs hindered further analysis of the T cells, but given the design of the FMPV-1 peptide vaccine which harbours predominantly CD4+ T cell epitopes and use of T cell proliferation protocol using incorporation of 3H-thymidine, 6 [26, 30] we hypothesise that the majority of the FMPV-1-specific T cells are CD4+, while not disregarding the possibility of FMPV-1-specific CD8+ T cells.
Given that healthy volunteers are immunocompetent, the results are nonetheless encouraging regarding immune response generation. This warrants further investigation in cancer patients, harbouring frameshift-mutated TGF-βR2 and other genes such as ASTE1 and TAF1 . In addition, as vaccines are likely to be most effective in low burden disease, prophylactic use of vaccines against the frameshift mutation transformation in Lynch carriers is a target for further research.
In conclusion, this novel approach for the treatment or prophylaxis of MSI-H/dMMR tumours using vaccination with FMPV-1/GM-CSF demonstrated a favourable safety and tolerability profile and generated a neoantigen-specific systemic immune response. These findings support further testing of FMPV-1/GM-CSF vaccination and support further clinical studies in cancer patients with frameshift mutations or as prophylaxis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to sincerely thank the volunteers who participated in this study and contributions from Litza McKenzie, Martina Fieldingova, Elizabeth Maria van Niekerk, Lena Chen, David Everton, Guy Fraser and Peter Green from Quotient Sciences Ltd.
Abbreviations
- AE
Adverse event
- cfDNA
Cell-free DNA
- dMMR
Deficiency in DNA mismatch repair
- DTH
Delayed type hypersensitivity
- ECG
Electrocardiogram
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- ID
Intradermal
- IU
International units
- MAPK
Mitogen-activated protein kinase
- MSI-H
Microsatellite instability high
- NE
Non-evaluable
- PBMCs
Peripheral blood mononuclear cells
- QTcF
Fridericia-corrected QT interval
- SI
Stimulation Index
- TGFβR2
Transforming growth factor β receptor 2
- ULN
Upper limit of normal
Author contributions
All authors reviewed the protocol and reviewed and revised the paper, contributed to and approved the final version for submission. RM, SA-B, HKE, EMI, BI and JAE designed the study; RM was the Medical Monitor; NS was the Principal Investigator; Quotient Sciences was involved in data acquisition and data analysis; RM and SA-B performed the interpretation of DTH and safety data; HKE performed the frameshift-mutated TGFβR2 cell-free DNA assays; EMI and HVJ performed T cell assays, data analysis and interpretation of T cell data; KRH performed quality check on T cell results and data analysis. Hubro Therapeutics provided IMP supply of FMPV-1 and GM-CSF.
Funding
This work was supported by Hubro Therapeutics AS and The Research Council of Norway, Project No.: 321590.
Availability of data and materials
All data generated or analysed during this study are included in the published article. Anonymised individual subject data are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
NS is an employee of Quotient Sciences which was contracted by Hubro Therapeutics AS to conduct the study. RMM, SA-B, HKE, BI, JAE and KRH were employed by study sponsor. EMI and HVJ are employees of Translational Research Unit, Section for Cellular Therapy, Department of Oncology, Oslo University Hospital—Radiumhospitalet, Oslo, Norway. The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval and consent to participate
This study was approved by the UK Medicines and Healthcare products Regulatory Agency and the protocol approved by the London – West London & Gene Therapy Advisory Committee Research Ethics Committee, the Norwegian National Research Ethics Committee and written informed consent was obtained from all subjects.
Consent for publication
The authors declare that they consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pinheiro M, Pinto C, Peixoto A et al (2015) Target gene mutational pattern in Lynch syndrome colorectal carcinomas according to tumour location and germline mutation. Br J Cancer 113(4):686–692. 10.1038/bjc.2015.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner S, Mullins CS, Linnebacher M (2018) Colorectal cancer vaccines: Tumor-associated antigens vs neoantigens. World J Gastroenterol 24(48):5418–5432. 10.3748/wjg.v24.i48.5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maby P, Galon J, Latouche JB (2016) Frameshift mutations, neoantigens and tumor-specific CD8(+) T cells in microsatellite unstable colorectal cancers. Oncoimmunology 5(5):e1115943. 10.1080/2162402X.2015.1115943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloor M, von Knebel DM (2016) The immune biology of microsatellite-unstable cancer. Trends Cancer 2(3):121–133. 10.1016/j.trecan.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 5.Saeterdal I, Bjorheim J, Lislerud K et al (2001) Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A 98(23):13255–13260. 10.1073/pnas.231326898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linnebacher M et al (2001) Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 93(1):6–11. 10.1002/ijc.1298. (PMID: 11391614) [DOI] [PubMed] [Google Scholar]
- 7.Sahin IH, Akce M, Alese O et al (2019) Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer 121(10):809–818. 10.1038/s41416-019-0599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillemanns P, Denecke A, Woelber L, Böhmer G, Jentschke M, Schjetne KW, Slot KMH, Fredriksen AB (2022) A therapeutic antigen-presenting cell-targeting DNA vaccine VB10.16 in HPV16-positive high-grade cervical intraepithelial neoplasia: results from a phase I/IIa Trial. Clin Cancer Res 28(22):4885 [DOI] [PubMed] [Google Scholar]
- 9.Palmer DH, Valle JW, Ma YT, Faluyi O, Neoptolemos JP, Gjertsen TJ, Iversen B, Eriksen JA, Møller AS, Aksnes AK, Miller RM, Dueland S (2020) TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas (CT TG01-01): a single-arm, phase 1/2 trial. Br J Cancer 122(7):971–977. 10.1038/s41416-020-0752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjeldsen JW et al (2021) A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med 27(12):2212–2223. 10.1038/s41591-021-01544-x. (Epub 2021 Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haakensen VD, Ojlert AK, Thunold S et al (2024) UV1 telomerase vaccine with ipilimumab and nivolumab as second line treatment for pleural mesothelioma—a phase II randomised trial. Eur J Cancer 202:113973 [DOI] [PubMed] [Google Scholar]
- 12.Nelde A, Rammensee HG, Walz JS (2021) The peptide vaccine of the future. Mol Cell Proteom 20:100022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disis ML, Schiffman K, Gooley TA, McNeel DG, Rinn K, Knutson KL (2000) Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin Cancer Res 6(4):1347–1350 [PubMed] [Google Scholar]
- 14.Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, Eriksen JA, Møller M, Baksaas I, Lothe RA, Saeterdal I, Gaudernack G (2001) Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 92(3):441–450. 10.1002/ijc.1205. (PMID: 11291084) [DOI] [PubMed] [Google Scholar]
- 15.Sæterdal I, Bjørheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Møller M, Lindblom A, Gaudernack G (2001) Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. PNAS 98(23):13255–13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sæterdal I, Gjertsen MK, Straten P, Eriksen JA, Gaudernack G (2002) A TGFβRII frameshift-mutation-derived CTL epitope recognized HLA-A2-restricted CD8+ T cells. Cancer Immunol Immunother 50:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, Von Knebel Doeberitz M (2001) Frameshift peptide-derived T-cell epitopes: A source of novel tumor-specific antigens. Int J Cancer 93:6–11 [DOI] [PubMed] [Google Scholar]
- 18.Roudko V, Cimen Bozkus C, Greenbaum B, Lucas A, Samstein R, Bhardwaj N (2021) Lynch syndrome and MSI-H cancers: from mechanisms to “off-the-shelf” cancer vaccines. Front Immunol 12:757804. 10.3389/fimmu.2021.757804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhurst RJ, Hata A (2012) Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 11(10):790–811. 10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz S, Wang J, Myeroff L et al (1995) Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268(5215):1336–1338. 10.1126/science.7761852 [DOI] [PubMed] [Google Scholar]
- 21.Fricke F, Mussack V, Buschmann D et al (2019) TGFBR2-dependent alterations of microRNA profiles in extracellular vesicles and parental colorectal cancer cells. Int J Oncol 55(4):925–937. 10.3892/ijo.2019.4859 [DOI] [PubMed] [Google Scholar]
- 22.Mittendorf EA, Clifton GT, Holmes JP et al (2012) Clinical trial results of HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients. Cancer 118(19):2594–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellingsen EB, O’Day S, Mezheyeuski A et al (2023) Clinical activity of combined telomerase vaccination and pembrolizumab in advanced melanoma: results from a phase I trial. Clin Cancer Res 29(16):3026–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu MT, Chang WC, Pao SC, Hung SI (2023) Delayed drug hypersensitivity reactions: molecular recognition, genetic susceptibility, and immune mediators. Biomedicines. 11(1):177. 10.3390/biomedicines11010177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbey C, Pradervand E, Barbier N et al (2007) Ex vivo monitoring of antigen-specific CD4+ T cells after recall immunization with tetanus toxoid. Clin Vaccine Immunol 14(9):1108–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Blasi D, Claessen I, Turksma AW et al (2020) Guidelines for analysis of low-frequency antigen-specific T cell results: Dye-based proliferation assay vs 3H-thymidine incorporation. J Immunol Methods 487:112907 [DOI] [PubMed] [Google Scholar]
- 27.Schultz HS, Reedtz-Runge SL, Bäckström BT et al (2017) Quantitative analysis of the CD4+ T cell response to therapeutic antibodies in helathy donors using a novel T cell:PBMC assay. PLoS ONE 12(5):e0178544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aamdal E, Inderberg EM, Ellingsen EB et al (2021) Combining a universal telomerase based cancer vaccine with ipilimumab in patients with metastatic melanoma – five-year follow up of a phase I/Iia Trial. Front Immunol 11(12):663865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallone R, Mannering SI, Brooks-Worrell BM et al (2011) Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol 163(1):33–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romar GA et al (2016) Research techniques made simple: techniques to assess cell proliferation. J Invest Dermatol 136(1):e1–e7. 10.1016/j.jid.2015.11.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in the published article. Anonymised individual subject data are available from the corresponding author on reasonable request.