Abstract
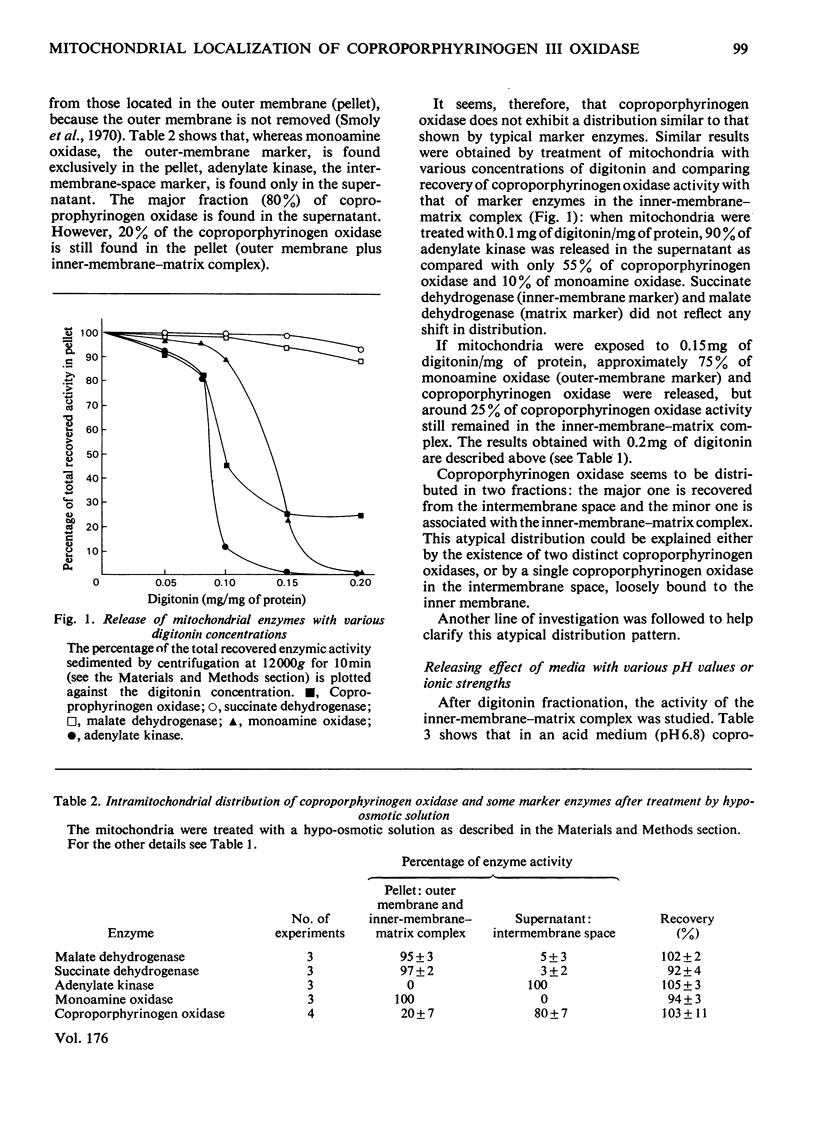
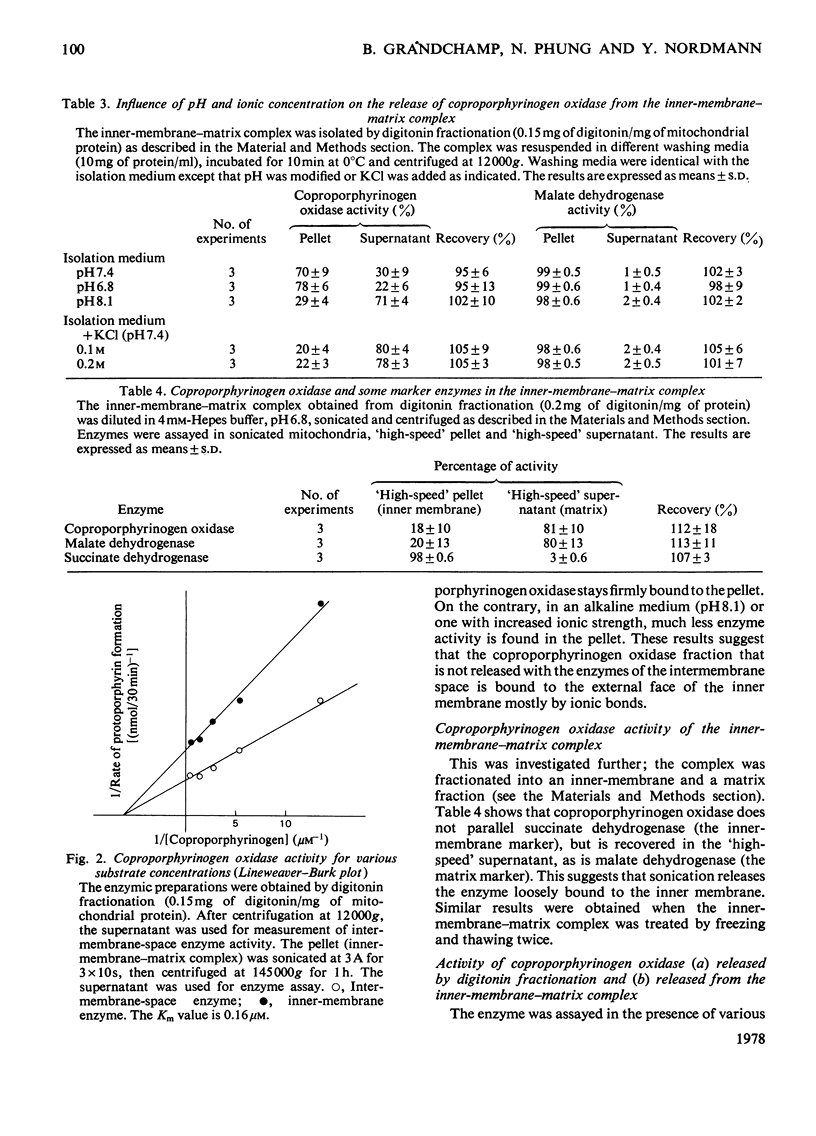
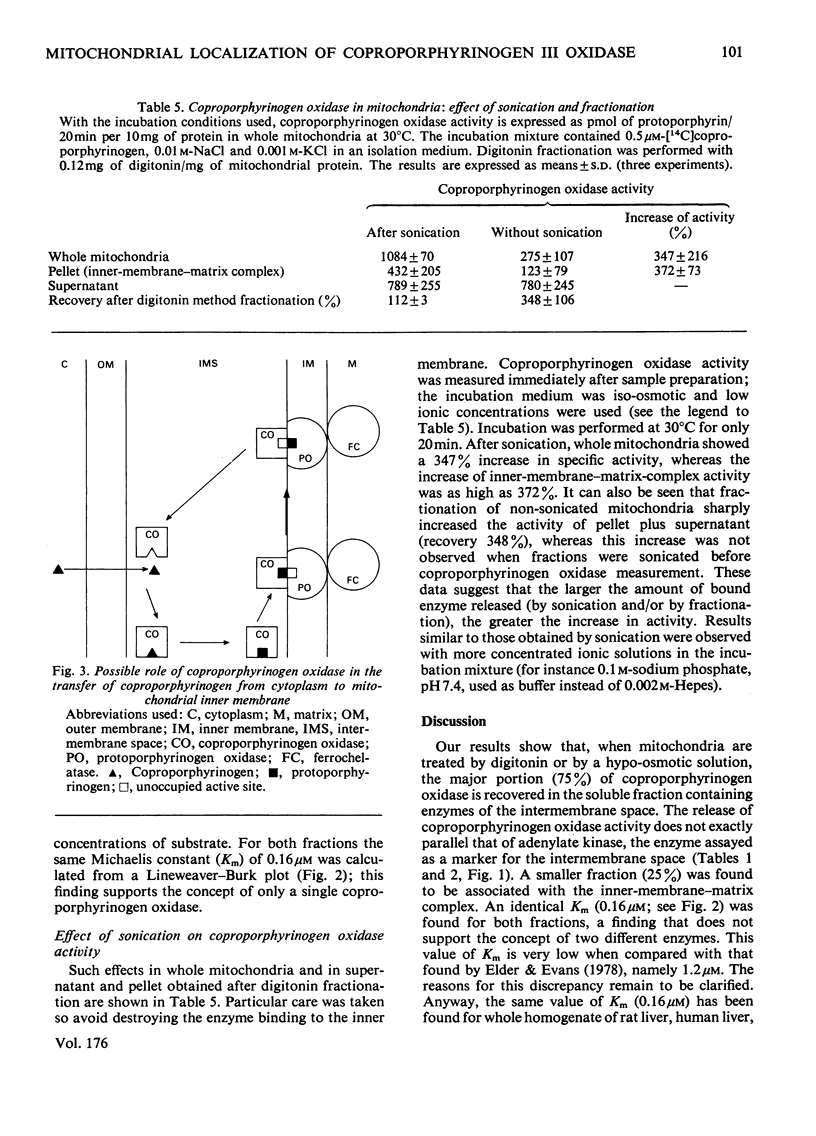
The location of coproporphyrinogen III oxidase in mitochondria was studied in rat liver by using the digitonin method or hypo-osmotic media for fractionation. The enzyme was found in the intermembrane space with a fraction loosely bound to the inner membrane. This fraction was released by washing the inner-membrane-matrix complex with alkaline solutions or solutions of high ionic strength. The enzyme in both fractions had the same Km (0.16 micrometer) for coproporphyrinogen III. When incubation was performed in a medium that avoided destruction of enzyme membrane binding, a dramatic increase in activity was observed after sonication of whole mitochondria or of the inner-membrane-matrix complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elder G. H., Evans J. O. A radiochemical method for the measurement of coproporphyrinogen oxidase and the utilization of substrates other than coproporphyrinogen III by the enzyme from rat liver. Biochem J. 1978 Jan 1;169(1):205–214. doi: 10.1042/bj1690205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Decreased lymphocyte coproporphyrinogen III oxidase activity in hereditary coproporphyria. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1089–1095. doi: 10.1016/0006-291x(77)91630-8. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lévy M., Toury R., André J. Séparation des membranes mitochondriales. Purification et caractérisation enzymatique de la membrane externe. Biochim Biophys Acta. 1967 Sep 9;135(4):599–613. doi: 10.1016/0005-2736(67)90092-2. [DOI] [PubMed] [Google Scholar]
- McEwen C. M., Jr Human plasma monoamine oxidase. 1. Purification and identification. J Biol Chem. 1965 May;240(5):2003–2010. [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J Biol Chem. 1975 Feb 25;250(4):1269–1274. [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoly J. M., Kuylenstierna B., Ernster L. Topological and functional organization of the mitochondrion. Proc Natl Acad Sci U S A. 1970 May;66(1):125–131. doi: 10.1073/pnas.66.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuyderhoudt F. M., Borst P., Huijing F. Intramitochondrial localization of 5-aminolaevulinate synthase induced in rat liver with allylisopropylacetamide. Biochim Biophys Acta. 1969 Apr 22;178(2):408–411. doi: 10.1016/0005-2744(69)90414-8. [DOI] [PubMed] [Google Scholar]


