Abstract
Abnormal fluctuations in thyroid function within the reference range were strongly associated with increased all-cause mortality. This study aimed to analyze the association between oxidative balance score (OBS) and free thyroxine (FT4) and thyrotropin (TSH) in euthyroid adults, as well as their interrelationships with mortality. 5727 euthyroid adults were selected from the National Health and Nutrition Examination Survey (NHANES). Weighted linear regression investigated the potential association of OBS with FT4 and TSH. In addition, COX proportional hazard models and restricted cubic spline (RCS) were used to investigate the association between OBS, FT4, TSH, and all-cause mortality. The results showed that OBS was negatively associated with serum FT4 concentrations in euthyroid adults (− 2.95%, 95% CI − 5.16%, − 0.92%). Additionally, the all-cause mortality rate was significantly higher in the fourth quartile (Q4) of FT4 compared to the first quartile (Q1) (HR 1.40, 95% CI 1.07–1.85). In the fourth quartile of OBS, the all-cause mortality rate was 31% lower than in Q1 (HR 0.69, 95% CI 0.52–0.92). Mediation analyses indicated that FT4 partially mediated the relationship between OBS and all-cause mortality. These results suggest a significant negative association between OBS and serum FT4, while both OBS and FT4 are strongly associated with mortality. However, the effect of OBS on serum FT4 is relatively limited, and therefore its clinical significance needs to be interpreted objectively.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-90491-5.
Keywords: Oxidative balance score, Thyrotropin, Free thyroxine, All-cause mortality, Diet, Lifestyle
Subject terms: Health care, Medical research
Introduction
Thyroid hormones (TH) play a pivotal role in mammals in various fields of growth and development, neural differentiation, and energy metabolism in the cardiovascular system1. The hypothalamic-pituitary-thyroid axis maintains normal thyroid function by regulating the concentration of thyroxine (T4) and thyroid-stimulating hormone (TSH) in the bloodstream1,2. The current clinical definition of normal thyroid function is primarily based on assessing thyroid function biomarkers within the normal reference range, typically the 2.5th to 97.5th percentile3,4. However, this approach to defining the range has certain limitations in practice, as it fails to consider the potential risks associated with clinical outcomes entirely. Several studies have demonstrated that fluctuations in serum FT4 and TSH levels may increase the risk of atrial fibrillation5, cardiovascular and cerebrovascular disease6,7, and mortality8, even within the reference range for thyroid function. Indeed, The development of various thyroid disorders is thought to be related to oxidative stress9–11.
Oxidative stress is characterized by an imbalance between the production of reactive oxygen species and the antioxidant defense system. This results in the disturbance of REDOX signaling and could potentially lead to harm at the molecular level, ultimately contributing to the onset of different diseases12–14. The Oxidative Balance Score (OBS) is a tool created for evaluating the oxidative stress conditions caused by diet and lifestyle, it is calculated based on a variety of dietary (pro-oxidant and antioxidant nutrients) and lifestyle exposures (smoking, alcohol consumption, obesity, and physical activity), a higher OBS indicating antioxidants are superior to pro-oxidants15. Several studies have shown that a reduction in OBS is linked to a higher likelihood of developing inflammatory and chronic diseases, as well as an increased risk of mortality from all causes16. However, few epidemiologic studies have explored the association between OBS and serum FT4, TSH in euthyroid adults.
Therefore, this study aimed to analyze the relationship between oxidative balance score (OBS) and thyroid function biomarkers among euthyroid adults and to further explore the interrelationships between OBS, FT4, TSH, and mortality.
Methods
Study design
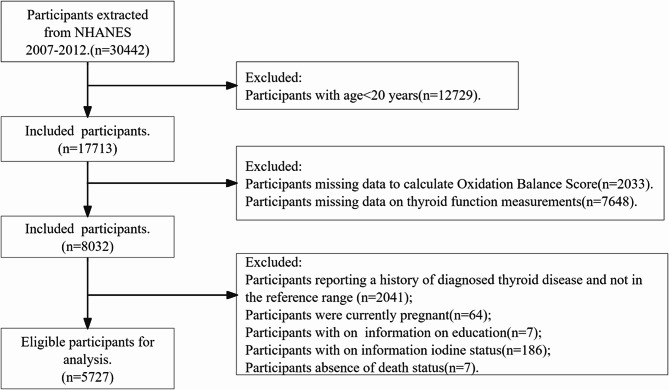
This research utilized information from the 2007–2012 National Health and Nutrition Examination Survey (NHANES), a cross-sectional study conducted by the National Centers for Disease Prevention17. Participants in the NHANES provided written informed consent and were approved by the National Center for Health Statistics Ethics Review Board18. Among the 30,442 subjects from 2007 to 2012, we excluded those who (1) were under the age of 20 (n = 12729), (2) had dietary (n = 1730) and lifestyle (303) OBS elemental deficiencies, (3) did not have thyroid function measurements (n = 7648), (4) reported a history of a previous diagnosis of thyroid disease (n = 750), (5) TSH not in the range of 0.4 to 4.5 mUI/L and FT4 concentrations not in the range of 9 to 25 pmol/L (n = 1291)19,20, (6) pregnant at the time the survey was conducted (n = 64), (7) missing education level (n = 7), (8) missing iodine status (n = 186), and (9) missing mortality status (n = 7). 5727 participants remain in the current analysis (Participant Flowchart, Fig. 1).
Fig. 1.
Flowchart for screening participants.
Oxidative balance score
The OBS was determined using a combination of 16 dietary nutrients and four lifestyle factors, encompassing 15 antioxidant elements and five pro-oxidant components15,21,22. Physical activity was assessed using the NHANES-recommended metabolic equivalent of task (MET) assignment, which includes one week of “(1) vigorous work-related activity, (2) moderate work-related activity, (3) walking or biking for transportation, (4) intense leisure-time physical activity, and (5) moderate leisure-time physical activity”. The resulting score was determined based on the activity level, with 0 points for low-intensity activity, 1 for moderate-intensity activity, and 2 for high-intensity activity. Participants were categorized into heavy drinkers (≥ 15 g/day for women and ≥ 30 g/day for men), non-heavy drinkers (0–15 g/day for women and 0–30 g/day for men), and non-drinkers, receiving 0, 1, and 2 points respectively23. The body mass index (BMI) was categorized based on the World Health Organization’s (WHO) classification for obesity (BMI ≥ 30 kg/m2), overweight (BMI = 25–30 kg/m2), and normal weight (BMI < 25 kg/m2), with corresponding scores of 0–2. The remaining OBS antioxidant factors included dietary fiber, carotenoids, riboflavin, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, and selenium. The factors were divided into three categories using a three-quartile approach, and the scores for each category (1–3) ranged from 0 to 2. Higher scores indicated an increase in antioxidant levels. The remaining pro-oxidant factors included total iron and fat. Unlike antioxidants, the top tertile of pro-oxidants received a score of 0, while the bottom tertile received a score of 2. The overall OBS was calculated by adding all the components together. For more information on how each element was scored, please see (Supplementary Table 1).
Measurement of serum FT4, TSH, and urinary iodine
Thyroid blood specimens were processed, stored and shipped to University of Washington, Seattle, WA. Detailed specimen collection and processing instructions are discussed in the NHANES Laboratory/Medical Technologists Procedures Manual (LPM) (https://www.cdc.gov/Nchs/Data/Nhanes/Public/2007/DataFiles/THYROD_E.htm). Using the Access HYPERsensitive human thyroid-stimulating hormone (hTSH) assay, which is a 3rd generation, two-site immunoenzymatic (“sandwich”) assay, to test the thyroid-stimulating hormone level. The Access FT4 assay is a two-step enzyme immunoassay. The amount of analyte in the sample is determined from a stored, multi-point calibration curve. Methodology details were described on the website (https://www.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2007). Concerning the clinical practice guidelines of the American Association of Clinical Endocrinologists, we defined a participant with normal thyroid function as having a serum TSH concentration of 0.4–4.5 mUI/L and an FT4 concentration of 9–25 pmol/L19,20. The iodine concentration in the urine was measured using mass spectrometry with inductively coupled plasma and dynamic reaction cells24.
Ascertainment of outcomes
The National Death Index (NDI) provided the mortality status and time of death for NHANES participants. The NCHS website provides corresponding methods for accessing this information (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm). The International Classification of Diseases, 10th edition (ICD-10), was used to establish the cause of death. This study examined all-cause mortality as the primary outcome.
Covariates
We incorporated variables that have been demonstrated to impact thyroid function and the risk of mortality in prior research, such as information on gender, age, race/ethnicity, level of education, status of iodine intake, and health condition. Participants’ iodine intake status was defined based on urinary iodine concentration (UIC), with UIC < 100 µg/L as iodine deficiency, 100–299 µg/L as usual, and ≥ 300 µg/L as iodine overdose25; the definition of Diabetes Mellitus (DM) included a physician’s report, glycosylated hemoglobin A1c (HbA1c) ≥ 6.5%, fasting blood glucose ≥ 7.0 µmol/L, or 120 min after an oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L. Hyperlipidemia is defined as total cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, LDL ≥ 130 mg/dL, or HDL < 40 mg/dL. The definition of hypertension includes self-reported diagnosis by a medical professional or an average of three consecutive measurements indicating systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. Cardiovascular disease was characterized by physician diagnoses obtained through personal interviews and included conditions such as coronary heart disease, angina, heart failure, heart disease, and stroke.
Statistical analyses
In performing the statistical analyses, we followed the CDC recommendations and chose the appropriate weights for the data analyses18. Descriptive analysis was conducted to examine the characteristics of participants, as well as their serum FT4, TSH levels, and overall health status. Continuous variables were presented as median values, while categorical variables were expressed as percentages. Multivariate linear regression models examined the association between OBS and FT4, TSH. Due to the skewed data distribution, natural logarithm conversion is performed for OBS and FT4, TSH. The results are then expressed as percent differences24. We selected the covariates for adjustment based on the findings from the previous NHANES study. Variance Inflation Factor (VIF) less than 10 was considered free of multicollinearity. We used Model 1 (unadjusted), Model 2 (adjusted for age, gender, and race), and Model 3 (adjusted for age, gender, race, education, and iodine status) to explore potential associations between OBS and normal thyroid function. The continuous OBS was converted into categorical variables using quartile methods, and the P value for trends was computed. Sensitivity analyses were performed to exclude participants with serum TPOAb (> 9 IU / mL) and TgAb (> 4 IU/mL) to minimize the effect of pre-existing immune disorders of thyroid tissue. In addition, we performed a stratified analysis. A multivariate COX proportional hazard model was employed to investigate the association between OBS, FT4, TSH, and all-cause mortality. Restricted cubic spline was employed to investigate the potential for a nonlinear relationship between these three variables and all-cause mortality. When examining the relationship between OBS and all-cause mortality, we also made adjustments for diabetes, hypertension, hyperlipidemia, and cardiovascular disease. In analyzing the association between FT4, TSH, and all-cause mortality, we further refined our model by including adjustments for smoking, alcohol consumption, and BMI. To assess whether the impact of OBS and all-cause mortality were influenced by FT4 or TSH, mediation effect analyses were conducted while controlling for the covariates, including age, sex, race, education, diabetes mellitus, hypertension, hyperlipidemia, and Cardiovascular disease26. The R software (version 4.2.2) and EmpowerStats were utilized for all statistical analyses. Statistical significance was determined based on a two-sided P-value below 0.05.
Results
Participant characteristics
The median age of the participants was 44 years (95% CI 31, 57). Approximately 51.7% of the participants were male, and non-Hispanic whites accounted for a larger proportion of the participants at about 67.8%. Regarding iodine intake, 17.3% of participants had excessive intake, while 34.3% had insufficient intake. Additionally, it was found that 46.7% of the participants were smokers. Furthermore, among the participants, 11.7%, 64.9%, 30.4%, and 5.92% had diabetes, hyperlipidemia, hypertension, and cardiovascular disease respectively. Over a median follow-up period of 133 months, it was observed that approximately 9.5% of the participants died. For more detailed characteristics of the participants, please refer to (Table 1).
Table 1.
Characteristics of study participants in NHANES 2007–2012.
| Characteristic | Weighted N = 76,849,583 Unweighted n = 5727 |
|---|---|
| Age | 44 (31, 57) |
| FT4 (pmol/L) | 10.30 (9.00, 11.60) |
| TSH (mUI/L) | 1.59 (1.11, 2.25) |
| OBS | 24 (18, 28) |
| Gender | |
| Male | 51.7% |
| Female | 48.3% |
| Race | |
| Mexican American | 8.9% |
| Other Hispanic | 5.6% |
| Non-Hispanic White | 67.8% |
| Non-Hispanic Black | 11.1% |
| Other Race | 6.6% |
| Education | |
| Less than 9th grade | 6.3% |
| 9–11th grade | 13.3% |
| High School Grad/GED or equivalent | 80.4% |
| UIC | |
| Iodine deficient (< 100 µg/L) | 34.3% |
| Normal (100–299 µg/L) | 48.4% |
| Excessive iodine intake(≥ 300 µg/L) | 17.3% |
| Smoking | |
| Yes | 46.7% |
| No | 53.3% |
| Drinking | |
| Yes | 22.6% |
| No | 72.3% |
| BMI | |
| Obesity | 33.2% |
| Overweight | 33.5% |
| Normal | 33.3% |
| Diabetes | |
| Yes | 11.7% |
| No | 88.3% |
| Hyperlipidemia | |
| Yes | 64.9% |
| No | 35.1% |
| Hypertension | |
| Yes | 30.4% |
| No | 69.6% |
| CVD | |
| Yes | 5.92 (%) |
| No | 94.08 (%) |
Median (IQR) or n (%).TSH, thyroid-stimulating hormone; FT4, free thyroxine; OBS, Oxidative Balance Score; BMI, body mass index; CVD, Cardiovascular disease; UIC stands for urine iodine concentration to evaluate iodine intake.
Association of OBS with FT4 and TSH in euthyroid participants
We explored the correlation between OBS and FT4, TSH by multivariate-adjusted linear regression analysis. After adjusting all confounding variables, the results indicated a notable inverse relationship between OBS and FT4 (− 2.95%, 95% CI − 5.16%, − 0.92%). Further, we divided OBS according to the quartile method. Compared to the lowest quantile of OBS, the serum FT4 in the third quantile of OBS decreased by 2.70% (− 4.91%, − 0.41%), and that in the fourth quantile of OBS decreased by 2.59% (− 4.81%, − 0.30%) (Table 2). The observed trend of decreasing FT4 with increasing OBS was statistically significant (p < 0.05). To validate the robustness of these results, we also performed sensitivity analyses by excluding individuals with serum TPOAb levels exceeding 9 IU/mL and TgAb levels surpassing 4 IU/mL. This was done to minimize the influence of pre-existing immune disorders that may impact thyroid tissue. These analyses’ findings aligned with our previous results (Supplementary Table S2). In addition, we separately assessed the association of dietary OBS and lifestyle OBS with serum FT4, and the results are shown in Supplementary Table S3. The results showed a significant negative association between dietary OBS and serum FT4 after adjusting for all covariates.
Table 2.
Adjusted percent difference (%) and 95% CI in serum thyroid function measures in relation to OBS among euthyroid participants 2007–2012.
| OBS | FT4% (95% CI) | TSH % (95% CI) |
|---|---|---|
| Model 1 | ||
| Continuous | − 1.60 (− 3.62,0.46)* | 9.14 (0.46,18.58) * |
| Q1 | Ref | Ref |
| Q2 | 1.13 (− 1.12,3.47) | − 2.28 (− 10.67,7.15) |
| Q3 | − 1.32 (− 3.53,0.93) | 7.89 (− 1.37,18.03) |
| Q4 | − 1.01 (− 3.17,1.18) | 5.68 (− 3.17,15.35) |
| P for trend | 0.089 | 0.053 |
| Model 2 | ||
| Continuous | − 2.50 (− 4.72, − 0.46) * | 5.68 (− 2.95,15.08) |
| Q1 | Ref | Ref |
| Q2 | 0.42 (− 1.83,2.80) | − 3.84 (− 12.10,5.20) |
| Q3 | − 2.37 (− 4.57, − 0.12) * | 3.51 (− 5.38,13.24) |
| Q4 | − 2.16 (− 4.35,0.09) * | 2.80 (− 6.03,12.46) |
| P for trend | 0.008 | 0.230 |
| Model 3 | ||
| Continuous | − 2.95 (− 5.16, − 0.92) * | 4.23 (− 4.50,13.76) |
| Q1 | Ref | Ref |
| Q2 | 0.28 (− 2.05,2.56) | − 4.28 (− 12.50,4.71) |
| Q3 | − 2.70 (− 4.91, − 0.41) * | 2.33 (− 6.46,12.20) |
| Q4 | − 2.59 (− 4.81, − 0.30) * | 1.62 (− 7.32,11.43) |
| P for trend | 0.003 | 0.361 |
FT4, free thyroxine; TSH, thyroid-stimulating hormone; Q, quartile; OBS, Oxidative Balance Score. Estimates are derived from a complex survey design, with three models utilized: Model 1 (unadjusted), Model 2 (adjusted for age, gender, and race), and Model 3 (adjusted for age, gender, race, education, and UIC). In the model, natural logarithm conversion was applied to adjust OBS and thyroid function indexes. The results were presented as the percentage difference in serum thyroid function per 10-unit increase in OBS. Percent differences = [e(ln10×β) – 1] × 100. *P < 0.05.
(Supplementary Table S4) displays an association between OBS and FT4 in various gender subcategories. In the model adjusting for all confounding, we observed that in male participants, OBS showed a more significant negative correlation with FT4, whereas in female participants, although the two showed the same tendency to be negatively correlated, the statistical significance of this association was relatively weak. When analyzing the interaction of gender on OBS, there was no interaction of gender on OBS (P for interaction > 0.05).
Association between OBS and all-cause mortality
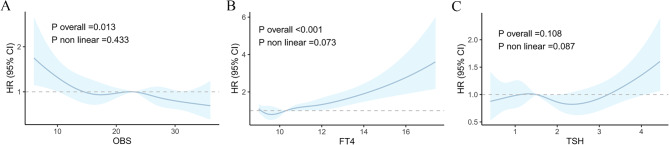
Our results indicated that, even after controlling for age, gender, race, education, diabetes, hyperlipidemia, hypertension, and cardiovascular disease, there was an inverse relationship between OBS and mortality. Participants with OBS in the highest percentile (75th–100th percentile) had a significant 31% lower risk of death compared to participants with OBS in the lowest percentile (0th-25th percentile). Restricted cubic spline plots provided a more visual representation (Table 3; Fig. 2A).
Table 3.
Association between OBS and all-cause mortality.
| HR (95% CI) | p-value | |
|---|---|---|
| OBS | ||
| Continuous | 0.98 (0.97,1.00) | 0.008 |
| 0–25 th | Ref | |
| 25–50th | 0.88 (0.69,1.13) | 0.308 |
| 50–75th | 0.87 (0.67,1.13) | 0.300 |
| 75–100th | 0.69 (0.52,0.92) | 0.012 |
HR, Hazard ratio; CI, confidence interval; OBS, oxidative balance score. Adjusted for age, gender, race, education, diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease.
Fig. 2.
(A) Dose-response relationship between OBS and all-cause mortality. (B) Dose-response relationship between FT4 and all-cause mortality. (C) Dose-response relationship between TSH and all-cause mortality. All models were adjusted for age, gender, race, education, diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease. The horizontal dotted line represents the hazard ratio of 1.
The associations between dietary OBS and lifestyle OBS and risk of death were assessed separately and the results are shown in Supplementary Table S5. The results showed that both dietary OBS and lifestyle OBS were negatively associated with all-cause mortality.
Correlation between indicators of thyroid function and all-cause mortality
In a COX proportional hazards model adjusting for age, gender, race, education, body mass index, smoking, alcohol consumption, diabetes, hyperlipidemia, hypertension, and cardiovascular disease, we observed a significant positive association between FT4 and mortality. In addition, we stratified FT4 according to quartiles, and participants with FT4 levels in the 75th–100th percentile had a substantial 40% increased risk of death compared with participants with FT4 levels in the 0th–25th percentile (P < 0.05). The Restricted Cubic Spline (RCS) further reinforces this finding, providing a more visual depiction of the trend (Table 4, Fig. 2B,C).
Table 4.
Association between FT4, TSH, and all-cause mortality.
| HR (95%CI) | p-value | |
|---|---|---|
| FT4 | ||
| Continuous | 1.13 (1.07,1.19) | < 0.001 |
| 0–25th | Ref | |
| 25–50th | 0.88 (0.68,1.14) | 0.340 |
| 50–75th | 1.19 (0.91,1.55) | 0.208 |
| 75–100th | 1.40 (1.07,1.85) | 0.016 |
| P for trend | < 0.001 | |
| TSH | ||
| Continuous | 1.04 (0.93,1.16) | 0.491 |
| 0–25th | Ref | |
| 25–50th | 0.98 (0.73,1.31) | 0.877 |
| 50–75th | 1.02 (0.76,1.35) | 0.916 |
| 75–100th | 0.99 (0.75,1.30) | 0.921 |
| P for trend | 0.822 | |
HR, Hazard ratio; CI, confidence interval; FT4, free thyroxine; TSH, thyroid-stimulating hormone; Adjusted for age, gender, race, education, smoking status, drinking status, BMI, diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease.
Association between OBS, FT4, TSH, and all-cause mortality
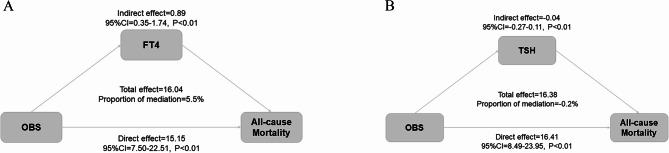
We performed a mediation effect analysis to examine whether there was a potential association between OBS and mortality mediated by thyroid function. After comprehensive adjustment for various confounders, it was observed that FT4 partially mediated between OBS and the risk of all-cause mortality, with a mediation effect share of 5.5% (p < 0.01) (Fig. 3A,B).
Fig. 3.
Mediation analysis for the associations between OBS and survival. FT4, free thyroxine; TSH, thyroid-stimulating hormone; OBS, Oxidative Balance Score. Adjusted for age, gender, race, education, diabetes mellitus, hypertension, hyperlipidemia, and cardiovascular disease.
Discussion
Based on a nationally representative survey of U.S. adults, we found a significant negative association between OBS and serum FT4 in euthyroid adults. When assessing the association between OBS, serum FT4, and all-cause mortality, the results showed that higher serum FT4 was significantly associated with an increased risk of death, and higher OBS was associated with a lesser risk of death. Mediation analysis showed that serum FT4 had a 5.5% mediating role in the potential association between OBS and all-cause mortality.
Negative correlation between OBS and FT4
This is the first cross-sectional study conducted in euthyroid participants to examine the association between diet and lifestyle related oxidative stress states and thyroid function. Our findings indicated a negative association between OBS and circulating FT4 levels, which remained consistent even after excluding participants with abnormal TPOAb or TgAb. Stratified analyses by gender showed that the relationship between OBS and FT4 was more statistically significant in male participants. This may be related to differences in men’s and women’s endocrine, metabolic, and immune systems27,28.
A cross-sectional study, encompassing individuals with normal and abnormal thyroid function, revealed a significant negative correlation between OBS and FT429, consistent with our findings. However, given that abnormal fluctuations in the normal range of thyroid function-related biomarkers are also strongly associated with mortality and that oxidative stress is not only a key factor in hypothyroidism but also constitutes one of the underlying pathologic processes in hyperthyroidism30–33, the present study focused on a group of subjects whose thyroid function was within the reference range. We performed a sensitivity analysis (excluding patients with positive thyroid peroxidase antibodies and positive thyroglobulin antibodies) to verify more precisely the association between OBS and FT4 and TSH levels in a population with normal thyroid function. In addition, a long-term cohort study of 324 euthyroid people showed that adherence to the Mediterranean dietary pattern reduced FT3 and FT4 levels in normal thyroid functioning people34. Meanwhile, the Dietary Antioxidant Index (CDAI), A comprehensive assessment of six dietary antioxidants, including vitamins A, C, and E, as well as manganese (Mn), selenium (Se), and zinc (Zn), was strongly associated with a significant reduction in FT4 levels in another cross-sectional study25. However, when the research focused on the complementary effects of nutrients, the results differed from what was expected. A randomized controlled trial showed that participants who received zinc (Zn) and selenium (Se) supplements for 8 weeks did not experience significant changes in their thyroid hormone levels compared with placebo35. In addition, animal experiments showed that the combined use of vitamin E and curcumin significantly alleviated the oxidative stress state in hyperthyroid rats and effectively regulated the symptoms associated with hyperthyroidism. In contrast, no significant improvement was observed when vitamin E or curcumin was given alone36,37. This highlights that nutrients do not exist in isolation but are absorbed and utilized by the body as the building blocks of the overall diet. Therefore, compared with supplementing a single nutrient, a comprehensive review and optimization of individual dietary patterns may be more effective in maintaining the stability and health of thyroid function. After all, the normal operation of thyroid function is inseparable from the fine regulation and dynamic balance of various trace elements, which promote the synthesis and metabolism of thyroid hormones. In addition, the link between lifestyle and thyroid hormone levels has also been established. A cross-sectional study of 5,766 participants clearly showed that smoking habits were significantly associated with an increase in FT4 levels38. At the same time, most studies report a negative association between BMI and FT4 levels39,40. However, regarding the specific effects of alcohol consumption and physical activity on thyroid function, there are still diverging results, and no unified conclusion has been reached41,42.
Antioxidant OBS reduces all-cause mortality
Prior epidemiological studies have consistently demonstrated a negative correlation between OBS and the risk of all-cause mortality, cardiovascular disease mortality, and cancer mortality43. Our study also found a notable link between OBS levels and all-cause mortality risk. Specifically, we observed that participants with higher OBS levels (antioxidant OBS) had a 31% lower risk of all-cause mortality than those with lower OBS levels (pro-oxidant OBS).
Lower FT4 levels in the reference range reduce all-cause mortality
Our study focused on participants whose thyroid function was within the normal reference range and found a significant association between their serum FT4 levels and all-cause mortality. Specifically, those individuals with serum FT4 in the 75th–100th percentile had a 40% increased risk of death compared to participants with serum FT4 in the 0th–25th percentile. This finding fits with previous studies, including a survey of U.S. adults that revealed a potential link between thyroid hormones and mortality in older adults, particularly in older adults with normal thyroid function, where lower FT4 levels were significantly associated with decreased mortality44. Thyroid hormones are critical to the function and metabolism of all organs and tissues and, in particular, have a complex impact on the cardiovascular system, which may be one of the reasons for this association. In addition, previous studies have noted that even elevated FT4 levels within the normal reference range are associated with an increased risk of hip fracture45, cardiovascular disease8, and atrial fibrillation46 in adults with normal thyroid function.
Based on these results, it is particularly important to maintain serum FT4 levels in the better health range for patients treated with levothyroxine (or thyroxine), as high serum FT4 may further increase the risk of death. There is clear evidence that levothyroxine treatment is associated with a strong dose-response relationship with a significant increase in the risk of fractures47. Also, studies have shown that levothyroxine use is significantly associated with an elevated risk of cancer, particularly brain, skin, pancreatic, and female breast cancers48. Similarly, although this study was limited by a database that failed to include patients with hyperthyroidism and patients treated with levothyroxine LT4 inhibition after thyroid cancer surgery, excessive serum FT4 in these populations is similarly potentially dangerous.
FT4 might play a role in the relationship between OBS and mortality
Thyroxine is a hormone with a wide range of physiologic effects and plays an important role in the functioning of all organ systems in the body. Studies have shown a significant correlation between high serum FT4 levels and increased mortality. Our findings suggest that OBS is strongly associated with changes in serum FT4 even within the reference range of thyroid function. FT4 mediated 5.5% of the effect of OBS on all-cause mortality. However, while there is an interesting and statistically significant relationship between OBS and serum FT4, the small effect of OBS on serum FT4 may have limited biological significance. We should objectively assess its clinical relevance.
Strengths and limitations
Our study has important strengths on several fronts. First, we focused on the association between variation in thyroid function within the reference range and risk of death, providing the first in-depth look at the association between OBS and people with normal thyroid function. In addition, the utilization of a large, nationally representative sample enhances the generalizability of our findings. Our study has some limitations. Although the findings suggest a statistically significant association between OBS and serum FT4, the magnitude of change in this association was relatively small, probably because we included only the population with normal thyroid function. Second, although we adjusted for multiple covariates and performed sensitivity analyses to assess the robustness of our results, there may have been other confounding factors that were not considered that could have affected our results.
Conclusion
In a nationally representative population, we found a significant negative association between OBS and serum FT4 in participants with normal thyroid function, while both OBS and FT4 were strongly associated with mortality. However, the effect of OBS on serum FT4 was relatively limited, and thus its clinical significance needs to be interpreted objectively. More studies are needed to validate our new findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, X.M., Z.Z., L.M., and Q.X. Methodology, X.M., Z.Z., L.M., and Q.X. Software, Q.X., and M.L. Validation, X.M., and L.M. Formal analysis, Q.X., B.Z., S.J. Data curation, L.M., H.G., and C.X. Writing—original draft preparation, Q.X., S.J., M.L., B.Z., Q.X., C.X., and H.G. Writing—review and editing, X.M., Z.Z., Q.X., and L.M. Investigation, X.M. Supervision, L.M. Funding acquisition, X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No. XJTU1AF-CRF-2023-020).
Data availability
This study analyzed publicly available data sets. These data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Declarations
Ethics approval and consent to participate
Participants in the NHANES provided written informed consent and were approved by the National Center for Health Statistics Ethics Review Board.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work.
Contributor Information
Lei Ma, Email: malei0214@126.com.
Xiaopeng Mei, Email: meixpxjtufh@126.com.
References
- 1.Stathatos, N. Thyroid physiology. Med. Clin. N. Am.96, 165–173. 10.1016/j.mcna.2012.01.007 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Choi, S. et al. Thyroxine-binding globulin, peripheral deiodinase activity, and thyroid autoantibody status in association of phthalates and phenolic compounds with thyroid hormones in adult population. Environ. Int.140, 105783. 10.1016/j.envint.2020.105783 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Ozarda, Y. et al. Distinguishing reference intervals and clinical decision limits—a review by the IFCC Committee on Reference Intervals and decision limits. Crit. Rev. Clin. Lab. Sci.55, 420–431. 10.1080/10408363.2018.1482256 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Xu, Y. N. et al. The optimal healthy ranges of thyroid function defined by the risk of cardiovascular disease and mortality: systematic review and individual participant data meta-analysis. Lancet Diabetes Endo. 11, 743–754. 10.1016/S2213-8587(23)00227-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgartner, C. et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation136, 2100–2116. 10.1161/CIRCULATIONAHA.117.028753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Tienhoven-Wind, L. J. & Dullaart, R. P. Low-normal thyroid function and novel cardiometabolic biomarkers. Nutrients7, 1352–1377. 10.3390/nu7021352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaker, L. et al. Thyroid function within the reference range and the risk of stroke: an Individual Participant Data Analysis. J. Clin. Endocrinol. Metab.101, 4270–4282. 10.1210/jc.2016-2255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu, Y. et al. The optimal healthy ranges of thyroid function defined by the risk of cardiovascular disease and mortality: systematic review and individual participant data meta-analysis. Lancet Diabetes Endocrinol.11, 743–754. 10.1016/S2213-8587(23)00227-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcocci, C., Leo, M. & Altea, M. A. Oxidative stress in graves’ disease. Eur. Thyroid J.1, 80–87. 10.1159/000337976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggeri, R. M. et al. Influence of dietary habits on oxidative stress markers in Hashimoto’s thyroiditis. Thyroid31, 96–105. 10.1089/thy.2020.0299 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Kubiak, K., Szmidt, M. K., Kaluza, J., Zylka, A. & Sicinska, E. Do dietary supplements affect inflammation, oxidative stress, and antioxidant status in adults with hypothyroidism or hashimoto’s disease?-A systematic review of controlled trials. Antioxid. (Basel). 1210.3390/antiox12101798 (2023). [DOI] [PMC free article] [PubMed]
- 12.Marrocco, I., Altieri, F. & Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Longev.2017 (6501046). 10.1155/2017/6501046 (2017). [DOI] [PMC free article] [PubMed]
- 13.Luo, J. et al. Age-related diseases and oxidative stress: what to do next? Ageing Res. Rev.57, 100982. 10.1016/j.arr.2019.100982 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Liguori, I. et al. Oxidative stress, aging, and diseases. Clin. Interv Aging. 13, 757–772. 10.2147/CIA.S158513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, W. et al. Association between the oxidative balance score and telomere length from the National Health and Nutrition Examination Survey 1999–2002. Oxid. Med. Cell. Longev.2022 (1345071). 10.1155/2022/1345071 (2022). [DOI] [PMC free article] [PubMed]
- 16.Talavera-Rodriguez, I. et al. Association between an oxidative balance score and mortality: a prospective analysis in the SUN cohort. Eur. J. Nutr.62, 1667–1680. 10.1007/s00394-023-03099-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie, Z. et al. Mediation of 10-year cardiovascular disease risk between inflammatory diet and handgrip strength: base on NHANES 2011–2014. Nutrients1510.3390/nu15040918 (2023). [DOI] [PMC free article] [PubMed]
- 18.Johnson, C. L. et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat.2, 1–24 (2013). [PubMed] [Google Scholar]
- 19.Garber, J. R. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American thyroid Association. Thyroid22, 1200–1235. 10.1089/thy.2012.0205 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Airaksinen, J. et al. Subclinical hypothyroidism and symptoms of depression: evidence from the National Health and Nutrition Examination Surveys (NHANES). Compr. Psychiatry. 109, 152253. 10.1016/j.comppsych.2021.152253 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Lee, J. H., Son, D. H. & Kwon, Y. J. Association between oxidative balance score and new-onset hypertension in adults: a community-based prospective cohort study. Front. Nutr.9, 1066159. 10.3389/fnut.2022.1066159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, X. et al. Interplay of sleep patterns and oxidative balance score on total cardiovascular disease risk: insights from the National Health and Nutrition Examination Survey 2005–2018. J. Glob Health. 13, 04170. 10.7189/jogh.14.04170 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu, Z. et al. Oxidative balance score was negatively associated with the risk of metabolic syndrome, metabolic syndrome severity, and all-cause mortality of patients with metabolic syndrome. Front. Endocrinol. (Lausanne). 14, 1233145. 10.3389/fendo.2023.1233145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun, Y. et al. Relationship between blood trihalomethane concentrations and serum thyroid function measures in U.S. adults. Environ. Sci. Technol.55, 14087–14094. 10.1021/acs.est.1c04008 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Liu, N., Ma, F., Feng, Y. & Ma, X. The association between the dietary inflammatory index and thyroid function in U.S. adult males. Nutrients13. 10.3390/nu13103330 (2021). [DOI] [PMC free article] [PubMed]
- 26.Imai, K., Keele, L. & Tingley, D. A general approach to causal mediation analysis. Psychol. Methods. 15, 309–334. 10.1037/a0020761 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Mammen, J. S. R. & Cappola, A. R. Autoimmune thyroid disease in women. JAMA325, 2392–2393. 10.1001/jama.2020.22196 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer, M., Glenn, T., Pilhatsch, M., Pfennig, A. & Whybrow, P. C. Gender differences in thyroid system function: relevance to bipolar disorder and its treatment. Bipolar Disord. 16, 58–71. 10.1111/bdi.12150 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Song, L. et al. Association between the oxidative balance score and thyroid function: results from the NHANES 2007–2012 and mendelian randomization study. PLoS One. 19, e0298860. 10.1371/journal.pone.0298860 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostami, R., Aghasi, M. R., Mohammadi, A. & Nourooz-Zadeh, J. Enhanced oxidative stress in Hashimoto’s thyroiditis: inter-relationships to biomarkers of thyroid function. Clin. Biochem.46, 308–312. 10.1016/j.clinbiochem.2012.11.021 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Ates, I. et al. The effect of oxidative stress on the progression of Hashimoto’s thyroiditis. Arch. Physiol. Biochem.124, 351–356. 10.1080/13813455.2017.1408660 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Rostami, R. et al. Serum selenium status and its interrelationship with serum biomarkers of thyroid function and antioxidant defense in Hashimoto’s thyroiditis. Antioxid. (Basel). 910.3390/antiox9111070 (2020). [DOI] [PMC free article] [PubMed]
- 33.Diana, T. et al. Stimulatory TSH-receptor antibodies and oxidative stress in graves disease. J. Clin. Endocrinol. Metab.103, 3668–3677. 10.1210/jc.2018-00509 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zupo, R. et al. Adherence to a mediterranean diet and thyroid function in obesity: a cross-sectional Apulian survey. Nutrients1210.3390/nu12103173 (2020). [DOI] [PMC free article] [PubMed]
- 35.Zavros, A. et al. The effects of zinc and selenium co-supplementation on resting metabolic rate, thyroid function, physical fitness, and functional capacity in overweight and obese people under a hypocaloric diet: a randomized, double-blind, and placebo-controlled trial. Nutrients1510.3390/nu15143133 (2023). [DOI] [PMC free article] [PubMed]
- 36.Subudhi, U., Das, K., Paital, B., Bhanja, S. & Chainy, G. B. Alleviation of enhanced oxidative stress and oxygen consumption of L-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem. Biol. Interact.173, 105–114. 10.1016/j.cbi.2008.02.005 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Subudhi, U. & Chainy, G. B. Expression of hepatic antioxidant genes in l-thyroxine-induced hyperthyroid rats: regulation by vitamin E and curcumin. Chem. Biol. Interact.183, 304–316 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Gruppen, E. G. et al. Cigarette smoking is associated with higher thyroid hormone and lower TSH levels: the PREVEND study. Endocrine67, 613–622. 10.1007/s12020-019-02125-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolffenbuttel, B. H. R. et al. Thyroid function and metabolic syndrome in the population-based LifeLines cohort study. BMC Endocr. Disord. 1710.1186/s12902-017-0215-1 (2017). [DOI] [PMC free article] [PubMed]
- 40.Knudsen, N. et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab.90, 4019–4024. 10.1210/jc.2004-2225 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Roa Duenas, O. H. et al. Thyroid function and physical activity: a population-based cohort study. Thyroid31, 870–875. 10.1089/thy.2020.0517 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Tian, L., Lu, C. & Teng, W. Association between physical activity and thyroid function in American adults: a survey from the NHANES database. BMC Public. Health. 24, 1277. 10.1186/s12889-024-18768-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez-Ruiz, A. et al. A review of a Priori defined oxidative balance scores relative to their components and impact on Health outcomes. Nutrients1110.3390/nu11040774 (2019). [DOI] [PMC free article] [PubMed]
- 44.Cappola, A. R. et al. Thyroid function in the euthyroid range and adverse outcomes in older adults. J. Clin. Endocrinol. Metab.100, 1088–1096. 10.1210/jc.2014-3586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson, J. et al. Higher serum free thyroxine levels are associated with increased risk of hip fractures in older men. J. Bone Miner. Res.39, 50–58. 10.1093/jbmr/zjad005 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaker, L. et al. Normal thyroid function and the risk of Atrial Fibrillation: the Rotterdam Study. J. Clin. Endocrinol. Metab.100, 3718–3724. 10.1210/jc.2015-2480 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Wu, C. C. et al. Risk of cancer in long-term levothyroxine users: retrospective population-based study. Cancer Sci.112, 2533–2541. 10.1111/cas.14908 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner, M. R. et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ342, d2238. 10.1136/bmj.d2238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study analyzed publicly available data sets. These data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.