Abstract
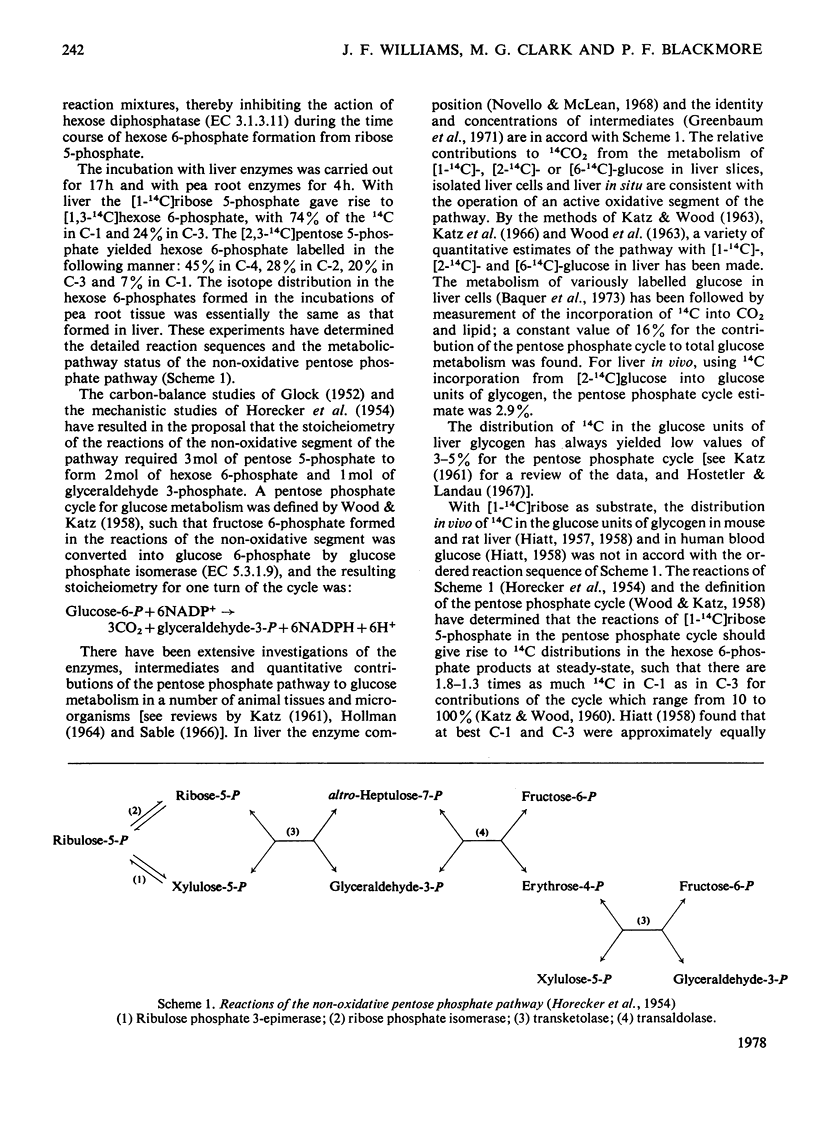
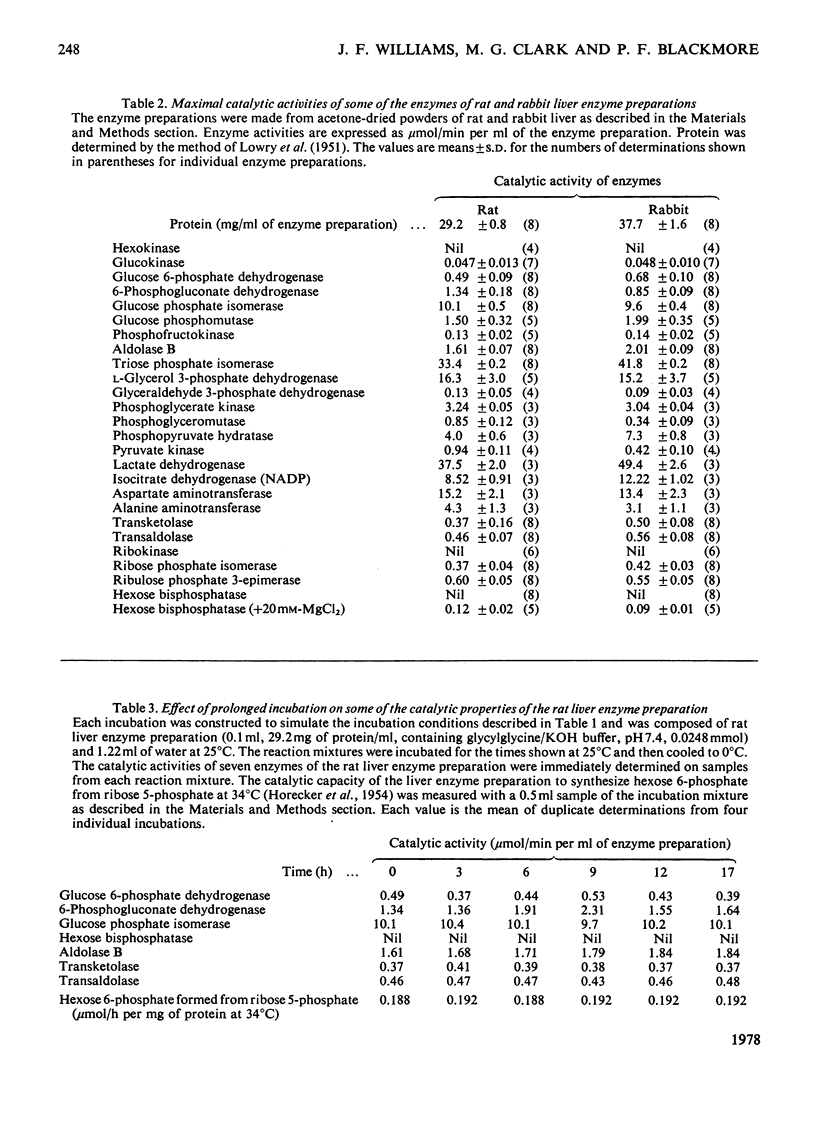
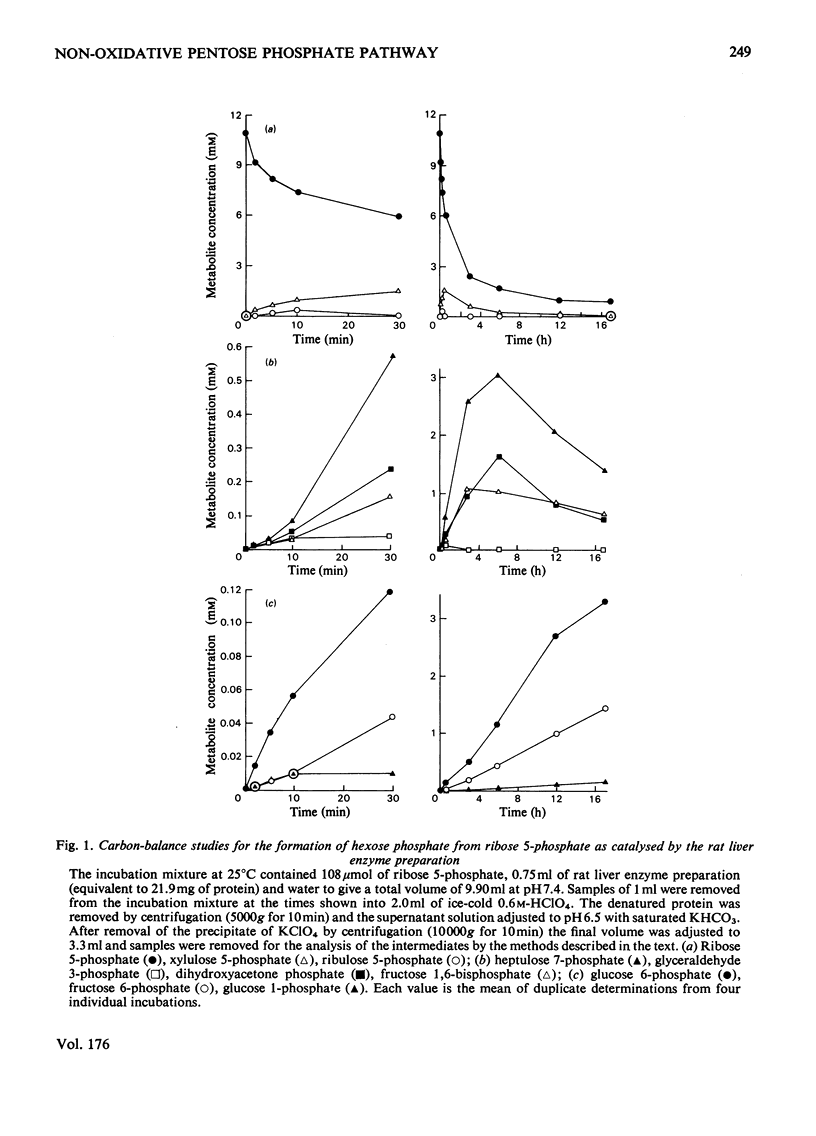
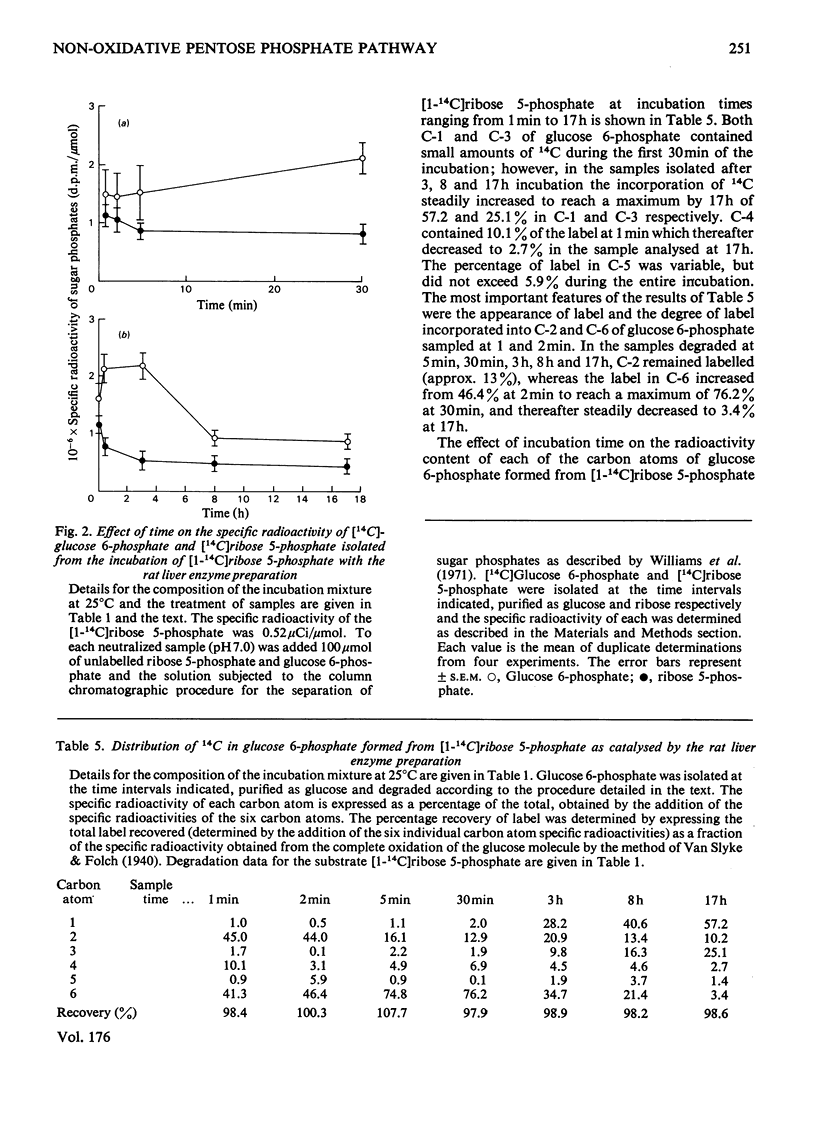
1. Glucose 5-phosphate was synthesized from ribose 5-phosphate by an enzyme extract prepared from an acetone-dried powder of rat liver. Three rates of ribose 5-phosphate utilization were observed during incubation for 17 h. An analysis of intermediates and products formed throughout the incubation revealed that as much as 20% of the substrate carbon could not be accounted for. 2. With [1-14C]ribose 5-phosphate as substrate, the specific radioactivity of [14C]glucose 6-phosphate formed was determined at 1, 2, 5 and 30 min and 3, 8 and 17 h. It increased rapidly to 1.9-fold the initial specific radioactivity of [1-14C]ribose 5-phosphate at 3 h and then decreased to a value approximately equal to that of the substrate at 6 h, and finally at 17 h reached a value 0.8-fold that of the initial substrate [1-14C]ribose 5-phosphate. 3. The specific radioactivity of [14C]ribose 5-phosphate decreased to approx. 50% of its inital value during the first 3 h of the incubation and thereafter remained unchanged. 4. The distribution of 14C in the six carbon atoms of [14C]glucose 6-phosphate formed from [1-14C]ribose 5-phosphate at 1, 2, 5 and 30 min and 3, 8 and 17 h was determined. The early time intervals (1--30 min) were characterized by large amounts of 14C in C-2 and in C-6 and with C-1 and C-3 being unlabelled. In contrast, the later time intervals (3--17 h) were characterized by the appearance of 14C in C-1 and C-3 and decreasing amounts of 14C in C-2 and C-6. 5. It is concluded that neither the currently accepted reaction sequence for the non-oxidative pentose phosphate pathway nor the 'defined' pentose phosphate-cycle mechanism can be reconciled with the labelling patterns observed in glucose 6-phosphate formed during the inital 3 h of the incubation.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., BANDURSKI R. S., GREINER C. M., JANG R. The metabolism of hexose and pentose phosphates in higher plants. J Biol Chem. 1953 Jun;202(2):619–634. [PubMed] [Google Scholar]
- BERNSTEIN I. A. Fermentation of ribose-C14 by Lactobacillus pentosus. J Biol Chem. 1953 Nov;205(1):309–316. [PubMed] [Google Scholar]
- BERNSTEIN I. A., LENTZ K., MALM M., SCHAMBYE P., WOOD H. G. Degradation of glucose-C14 with Leuconostoc mesenteroides; alternate pathways and tracer patterns. J Biol Chem. 1955 Jul;215(1):137–152. [PubMed] [Google Scholar]
- Baquer N. Z., Cascales M., Teo B. C., McLean P. The activity of the pentose phosphate pathway in isolated liver cells. Biochem Biophys Res Commun. 1973 May 1;52(1):263–269. doi: 10.1016/0006-291x(73)90982-0. [DOI] [PubMed] [Google Scholar]
- Beevers H. Intermediates of the Pentose Phosphate Pathway as Respiratory Substrates. Plant Physiol. 1956 Sep;31(5):339–347. doi: 10.1104/pp.31.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Williams J. F., Blackmore P. F. The transketolase exchange reaction in vitro. Biochem J. 1971 Nov;125(1):381–384. doi: 10.1042/bj1250381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Williams J. F., Kolos G., Hickie J. B. The measurement of transketolase activity in heart preparations. Experientia. 1972 May 15;28(5):613–615. doi: 10.1007/BF01931910. [DOI] [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. Pentose phosphate isomerase and epimerase from animal tissues. Biochem J. 1956 Nov;64(3):567–578. doi: 10.1042/bj0640567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENBERG F., Jr, DAYTON P. G., BURNS J. J. Studies on the glucuronic acid pathway of glucose metabolism. J Biol Chem. 1959 Feb;234(2):250–253. [PubMed] [Google Scholar]
- GIBBS M., HORECKER B. L. The mechanism of pentose phosphate conversion to hexose monophosphate. II. With pea leaf and pea root preparations. J Biol Chem. 1954 Jun;208(2):813–820. [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E. The formation and breakdown of pentophosphates by liver fractions. Biochem J. 1952 Dec;52(4):575–583. doi: 10.1042/bj0520575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- HIATT H. H. Glycogen formation via the pentose phosphate pathway in mice in vivo. J Biol Chem. 1957 Feb;224(2):851–859. [PubMed] [Google Scholar]
- HIATT H. H. Studies of ribose metabolism. III. The pathway of ribose carbon conversion to glucose in man. J Clin Invest. 1958 May;37(5):651–654. doi: 10.1172/JCI103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORECKER B. L., GIBBS M., KLENOW H., SMYRNIOTIS P. Z. The mechanism of pentose phosphate conversion to hexose monophosphate. I. With a liver enzyme preparation. J Biol Chem. 1954 Mar;207(1):393–403. [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., SEEGMILLER J. E. The enzymatic conversion of 6-phosphogluconate to ribulose-5-phosphate and ribose-5-phosphate. J Biol Chem. 1951 Nov;193(1):383–396. [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Williams J. F. Kinetic studies on the regulation of rabbit liver pyruvate kinase. Biochem J. 1973 Feb;131(2):287–301. doi: 10.1042/bj1310287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ J., ABRAHAM S., HILL R., CHAIKOFF I. L. The occurrence and mechanism of the hexose monophosphate shunt in rat liver slices. J Biol Chem. 1955 Jun;214(2):853–868. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1963 Feb;238:517–523. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of glucose-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1960 Aug;235:2165–2177. [PubMed] [Google Scholar]
- KITOS P. A., WANG C. H., MOHLER B. A., KING T. E., CHELDELIN V. H. Glucose and gluconate dissimilation in Acetobacter suboxydans. J Biol Chem. 1958 Dec;233(6):1295–1298. [PubMed] [Google Scholar]
- KORKES S. Carbohydrate metabolism. Annu Rev Biochem. 1956;25:685–734. doi: 10.1146/annurev.bi.25.070156.003345. [DOI] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J. 1968 May;107(6):775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. The mechanism of the non-oxidative segment of the pentose cycle in the liver. Biochem Biophys Res Commun. 1974 Nov 27;61(2):774–780. doi: 10.1016/0006-291x(74)91024-9. [DOI] [PubMed] [Google Scholar]
- SABLE H. Z. Pentose metabolism in extracts of yeast and mammalian tissues. Biochim Biophys Acta. 1952 Jun;8(6):687–697. doi: 10.1016/0006-3002(52)90106-6. [DOI] [PubMed] [Google Scholar]
- SHARMA C., MANJESHWAR R., WEINHOUSE S. EFFECTS OF DIET AND INSULIN ON GLUCOSE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES OF RAT LIVER. J Biol Chem. 1963 Dec;238:3840–3845. [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable H. Z. Biosynthesis of ribose and deoxyribose. Adv Enzymol Relat Areas Mol Biol. 1966;28:391–460. doi: 10.1002/9780470122730.ch7. [DOI] [PubMed] [Google Scholar]
- TABACHNICK M., SRERE P. A., COOPER J., RACKER E. The oxidative pentose phosphate cycle. III. The interconversion of ribose 5-phosphate, ribulose 5-phosphate and xylulose 5-phosphate. Arch Biochem Biophys. 1958 Apr;74(2):315–325. doi: 10.1016/0003-9861(58)90003-1. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- WASHKO M. E., RICE E. W. Determination of glucose by an improved enzymatic procedure. Clin Chem. 1961 Oct;7:542–545. [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- WOOD H. G., KATZ J. The distribution of C14 in the hexose phosphates and the effect of recycling in the pentose cycle. J Biol Chem. 1958 Dec;233(6):1279–1282. [PubMed] [Google Scholar]
- Williams J. F., Blackmore P. F., Clark M. G. New reaction sequences for the non-oxidative pentose phosphate pathway. Biochem J. 1978 Oct 15;176(1):257–282. doi: 10.1042/bj1760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Rienits K. G., Schofield P. J., Clark M. G. The pentose phosphate pathway in rabbit liver. Studies on the metabolic sequence and quantitative role of the pentose phosphate cycle by using a system in situ. Biochem J. 1971 Aug;123(5):923–943. doi: 10.1042/bj1230923. [DOI] [PMC free article] [PubMed] [Google Scholar]


