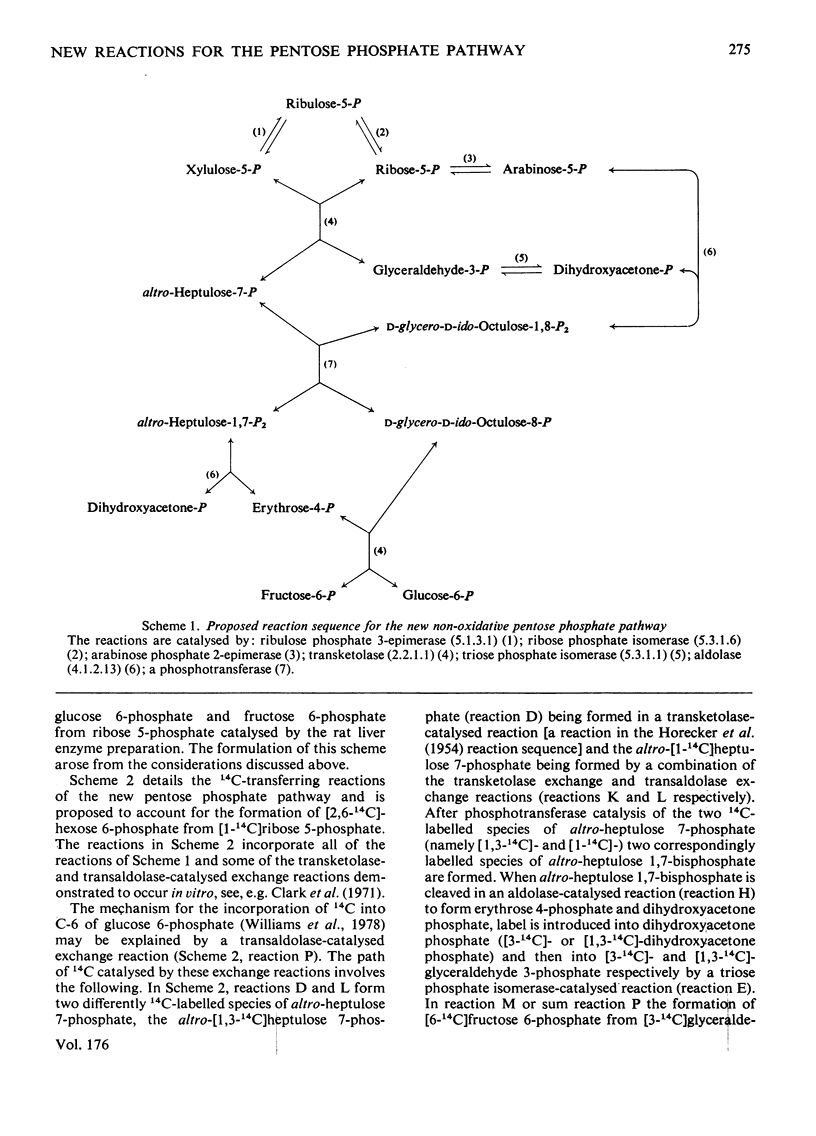
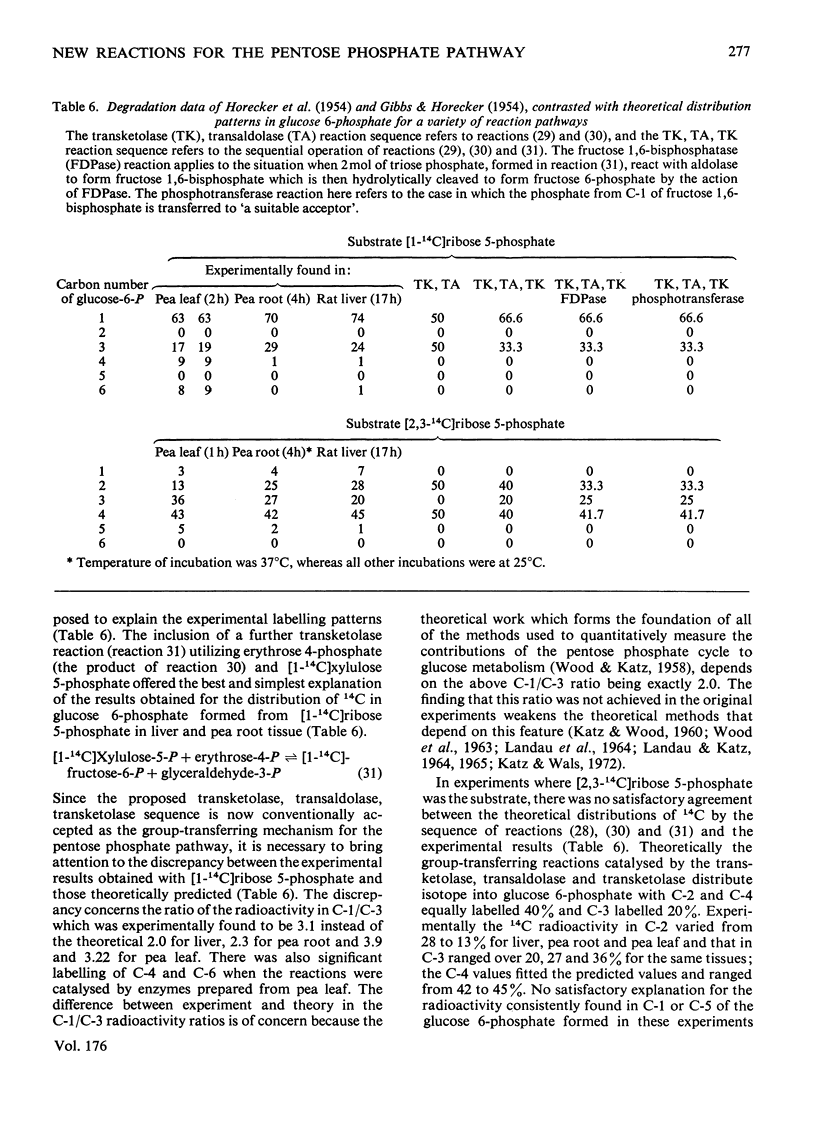
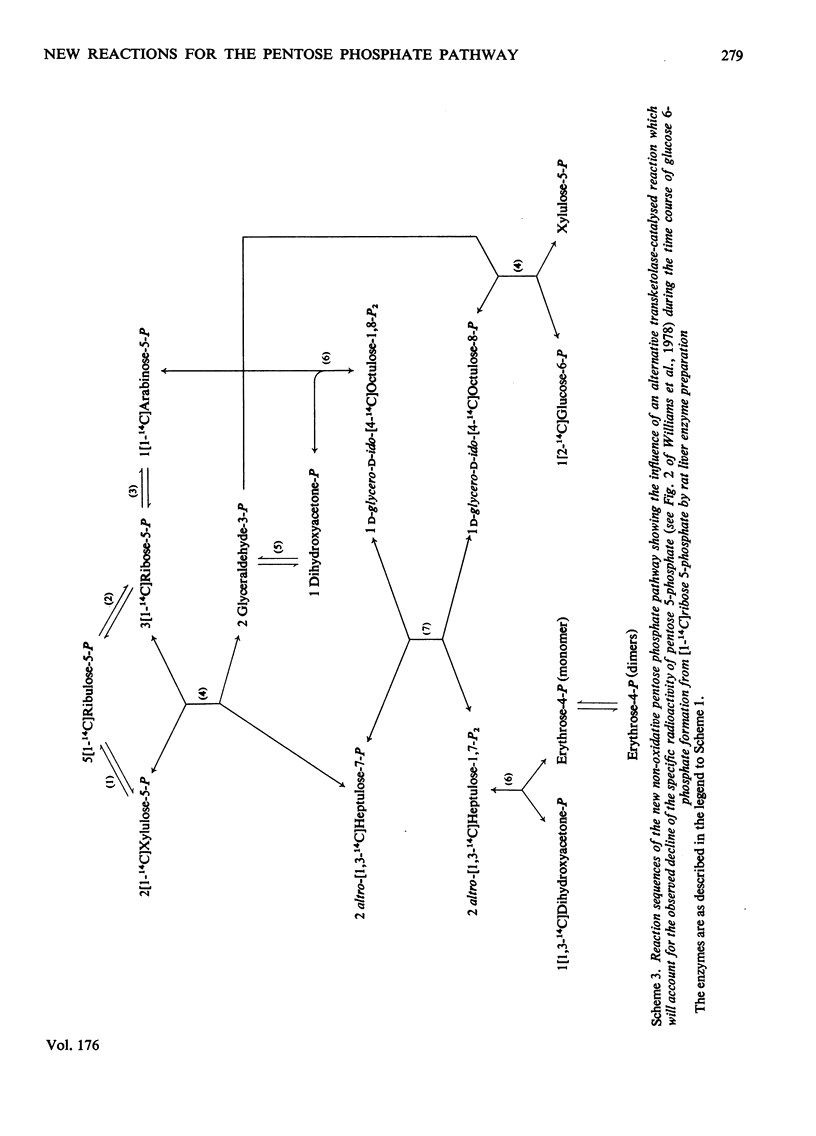
Abstract
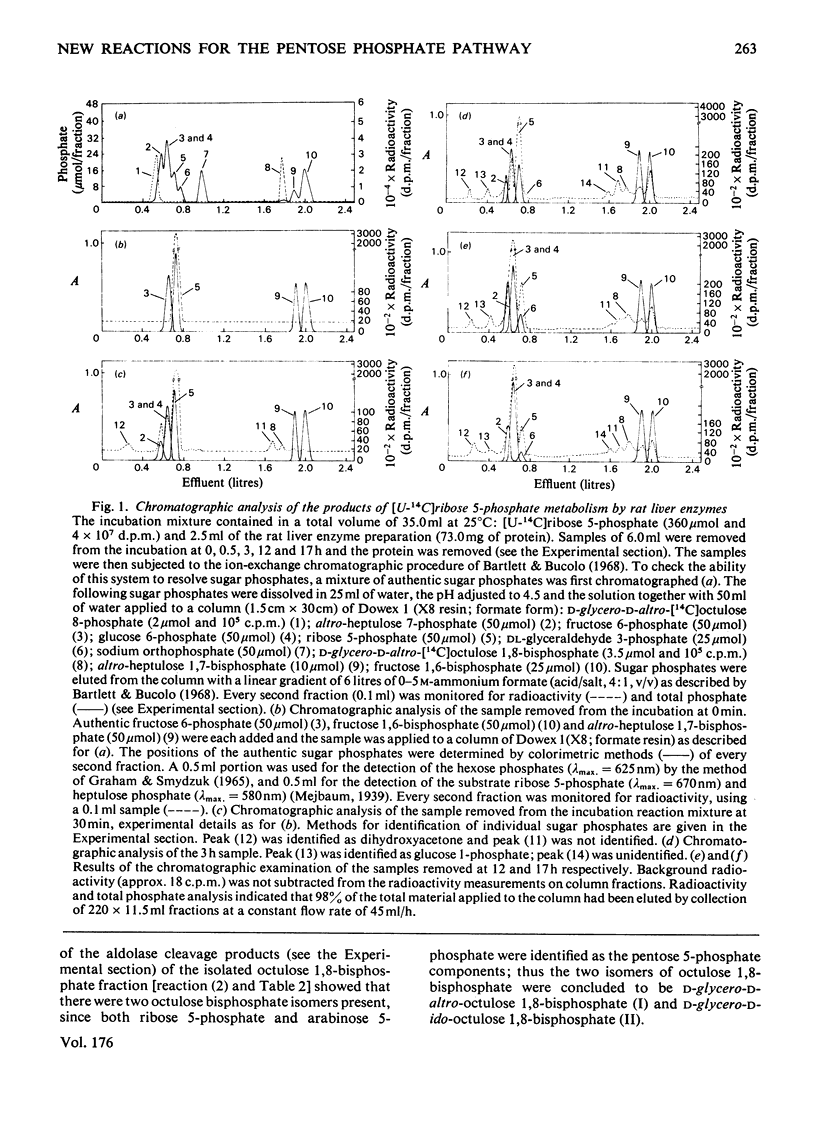
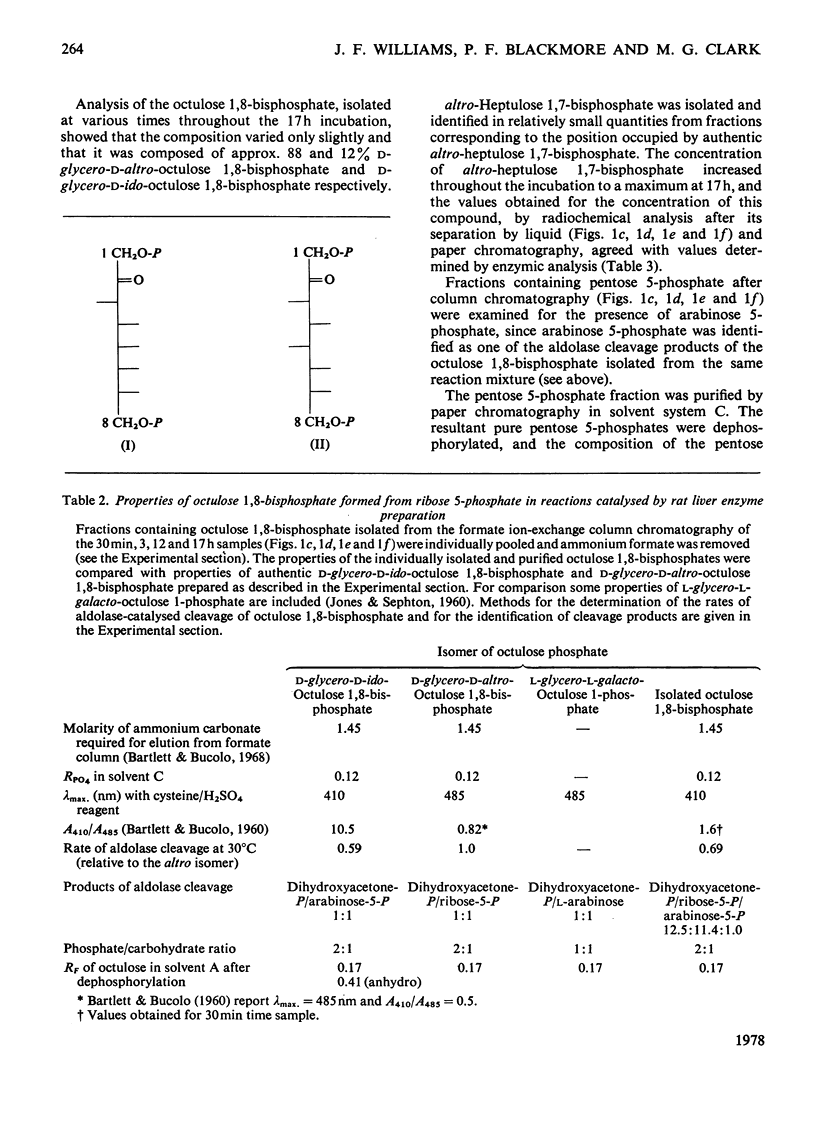
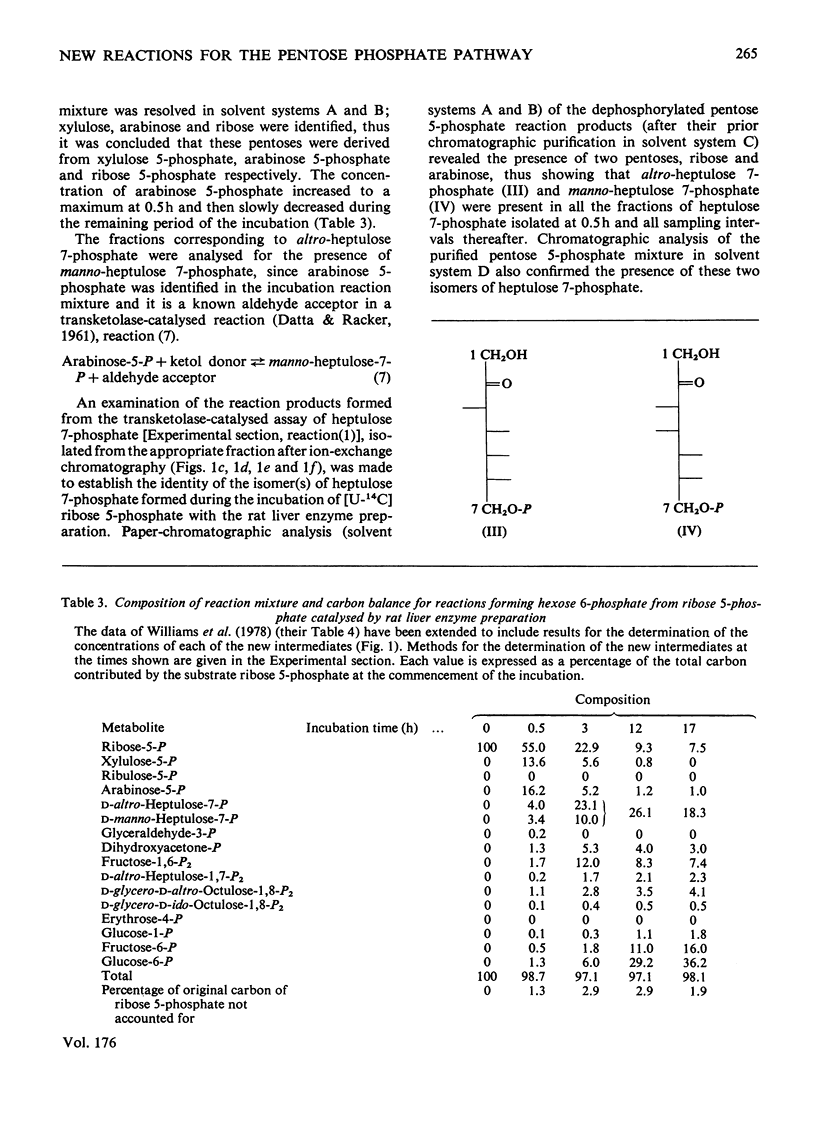
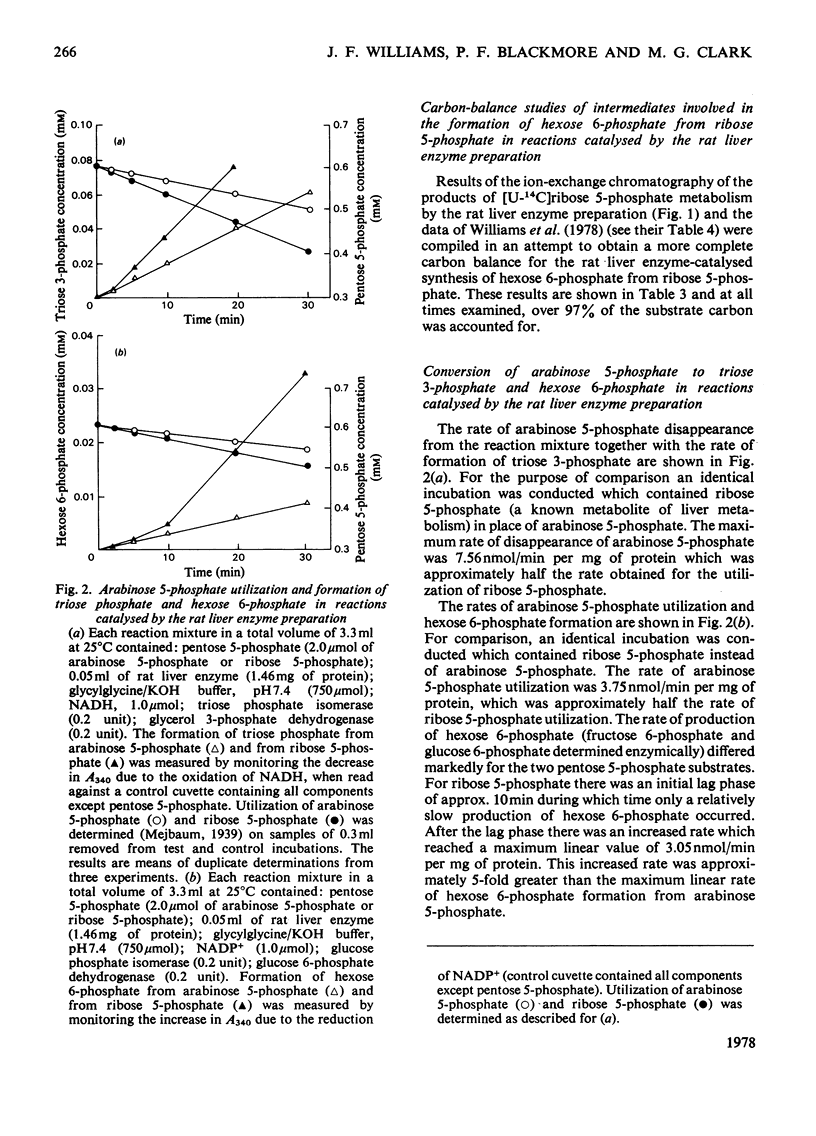
1. Reactions leading to the formation of 14C-labelled volatile compounds and compounds volatile under acid conditions were investigated in a system actively synthesizing hexose 6-phosphates from [U-14C]ribose 5-phosphate by reactions catalysed by enzymes prepared from acetone-dried powder of rat liver; no reactions involving 14C-labelled volatile compounds were detected. Similarly the fixation of 14C-labelled volatile compounds into hexose 6-phosphate could not be detected. 2. A complete carbon balance was made for the reactants, intermediates and products of the reactions involved in the conversion of ribose 5-phosphate into hexose 6-phosphate by enzymes of rat liver. Five additional intermediates of pentose 5-phosphate metabolism in liver were detected, namely D-manno-heptulose 7-phosphate, D-altro-heptulose 1,7-bisphosphate, D-glycero-D-ido-octulose 1,8-bisphosphate, D-glycero-D-altro-octulose 1,8-bisphosphate and D-arabinose 5-phosphate. 3. D-Arabinose 5-phosphate was found to be utilized by a rat liver enzyme preparation to produce both hexose 6-phosphate and triose phosphate. 4. D-Arabinose 5-phosphate was reversibly converted into other pentose 5-phosphates. Paper chromatographic and enzymic evidence indicated that the conversion involved an enzyme tentatively named arabinose phosphate 2-epimerase, which catalyses the following reaction: D-arabinose 5-P in equilibrium D-ribose-5-P. 5. A variety of rat tissues also utilized D-arabinose 5-phosphate to produce both hexose 6-phosphate and triose phosphate and at a rate comparable with that obtained with D-ribose 5-phosphate. 6. A new reaction sequence for the non-oxidative pentose phosphate pathway in liver is proposed.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R., BUCOLO G. Octulose phosphates from the human red blood cell. Biochem Biophys Res Commun. 1960 Nov;3:474–478. doi: 10.1016/0006-291x(60)90158-3. [DOI] [PubMed] [Google Scholar]
- BAXTER J. N., PERLIN A. S., SIMPSON F. J. Preparation and assay of D-erythrose 4-phosphate. Can J Biochem Physiol. 1959 Feb;37(2):199–209. [PubMed] [Google Scholar]
- Bartlett G. R., Bucolo G. The metabolism of ribonucleoside by the human erythrocyte. Biochim Biophys Acta. 1968 Mar 11;156(2):240–253. doi: 10.1016/0304-4165(68)90253-5. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Williams J. F., MacLeod J. K. Dimerization of erythrose 4-phosphate. FEBS Lett. 1976 Apr 15;64(1):222–226. doi: 10.1016/0014-5793(76)80288-8. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., SCOTT D. B. M. Formation of pentose phosphate from 6-phosphogluconate. Science. 1950 May 19;111(2890):543–544. doi: 10.1126/science.111.2890.543. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Williams J. F., Blackmore P. F. The transketolase exchange reaction in vitro. Biochem J. 1971 Nov;125(1):381–384. doi: 10.1042/bj1250381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA A. G., RACKER E. Mechanism of action of transketolase. I. Properties of the crystalline yeast enzyme. J Biol Chem. 1961 Mar;236:617–623. [PubMed] [Google Scholar]
- DISCHE Z., IGALS D. Mechanisms in the interconversion of ribose 5-phosphate and hexose 6-phosphate in human hemolyzates. II. Erythrose 4-phosphate as intermediate and rate regulator in the interconversion of ribose 5-phosphate and hexose 6-phosphate. Arch Biochem Biophys. 1961 May;93:201–210. doi: 10.1016/0003-9861(61)90250-8. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., SHIGEURA H. T., LANDSBERG E. Mechanisms in the interconversion of ribose 5-phosphate and hexose 6-phosphate in human hemolyzates. 1. Sedohetulose and triose phosphates as intermediates in the conversion of ribose 5-phosphate to hexose 6-phosphate in human hemolyzates. Arch Biochem Biophys. 1960 Jul;89:123–133. doi: 10.1016/0003-9861(60)90022-9. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F. Oxidation of phosphohexonate and pentose phosphoric acids by yeast enzymes: Oxidation of phosphohexonate. II. Oxidation of pentose phosphoric acids. Biochem J. 1938 Sep;32(9):1626–1644. doi: 10.1042/bj0321626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M., HORECKER B. L. The mechanism of pentose phosphate conversion to hexose monophosphate. II. With pea leaf and pea root preparations. J Biol Chem. 1954 Jun;208(2):813–820. [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Levels of enzymes of the direct oxidative pathway of carbohydrate metabolism in mammalian tissues and tumours. Biochem J. 1954 Jan;56(1):171–175. doi: 10.1042/bj0560171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D., Smydzuk J. Use of anthrone in the quantitative determination of hexose phosphates. Anal Biochem. 1965 May;11(2):246–255. doi: 10.1016/0003-2697(65)90012-6. [DOI] [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- Gumaa K. A., McLean P. The pentose phosphate pathway of glucose metabolism. Enzyme profiles and transient and steady-state content of intermediates of alternative pathways of glucose metabolism in Krebs ascites cells. Biochem J. 1969 Dec;115(5):1009–1029. doi: 10.1042/bj1151009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., Mclean P. Effect of insulin and diet on the steady state concentrations of intermediates of the pentose phosphate pathway of glucose metabolism in liver. FEBS Lett. 1968 Sep;1(4):227–229. doi: 10.1016/0014-5793(68)80068-7. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., GIBBS M., KLENOW H., SMYRNIOTIS P. Z. The mechanism of pentose phosphate conversion to hexose monophosphate. I. With a liver enzyme preparation. J Biol Chem. 1954 Mar;207(1):393–403. [PubMed] [Google Scholar]
- HORECKER B. L., MEHLER A. H. Carbohydrate metabolism. Annu Rev Biochem. 1955;24:207–274. doi: 10.1146/annurev.bi.24.070155.001231. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z. Purification and properties of yeast transaldolase. J Biol Chem. 1955 Feb;212(2):811–825. [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of glucose-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1960 Aug;235:2165–2177. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Pentose cycle and reducing equivalents in rat mammary-gland slices. Biochem J. 1972 Jul;128(4):879–899. doi: 10.1042/bj1280879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman F. C., Harkonen M. H. Metabolites and enzymes of the pentose phosphate pathway in isolated nerve endings. J Neurochem. 1977 Apr;28(4):745–750. doi: 10.1111/j.1471-4159.1977.tb10622.x. [DOI] [PubMed] [Google Scholar]
- LANDAU B. R., BARTSCH G. E., KATZ J., WOOD H. G. ESTIMATION OF PATHWAY CONTRIBUTIONS TO GLUCOSE METABOLISM AND OF THE RATE OF ISOMERIZATION OF HEXOSE 6-PHOSPHATE. J Biol Chem. 1964 Mar;239:686–696. [PubMed] [Google Scholar]
- LANDAU B. R., KATZ J. A QUANTITATIVE ESTIMATION OF THE PATHWAYS OF GLUCOSE METABOLISM IN RAT ADIPOSE TISSUE IN VITRO. J Biol Chem. 1964 Mar;239:697–704. [PubMed] [Google Scholar]
- LEVIN D. H., RACKER E. Condensation of arabinose 5-phosphate and phosphorylenol pyruvate by 2-keto-3-deoxy-8-phosphooctonic acid synthetase. J Biol Chem. 1959 Oct;234:2532–2539. [PubMed] [Google Scholar]
- LJUNGDAHL L., WOOD H. G., RACKER E., COURI D. Formation of unequally labeled fructose 6-phosphate by an exchange reaction catalyzed by transaldolase. J Biol Chem. 1961 Jun;236:1622–1625. [PubMed] [Google Scholar]
- MORTON R. K. The phosphotransferase activity of phosphatases. 1. Spectrophotometric methods for the estimation of some phosphate esters and other compounds. Biochem J. 1958 Sep;70(1):134–139. doi: 10.1042/bj0700134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The phosphotransferase activity of phosphatases. 2. Studies with purified alkaline phosphomonoesterases and some substrate-specific phosphatases. Biochem J. 1958 Sep;70(1):139–150. doi: 10.1042/bj0700139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The phosphotransferase activity of phosphatases. 3. Comparison of enzymic catalysis by acid phosphatase with nonenzymic catalysis at acid pH values. Biochem J. 1958 Sep;70(1):150–155. doi: 10.1042/bj0700150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novello F., McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J. 1968 May;107(6):775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTREMOLI S., BONSIGNORE A., GRAZI E., HORECKER B. L. A coupled reaction catalyzed by the enzymes transketolase and transaldolase. J Biol Chem. 1960 Jul;235:1881–1887. [PubMed] [Google Scholar]
- Peterkofsky A., Racker E. The reductive pentose phosphate cycle. III. Enzyme activities in cell-free extracts of photosynthetic organisms. Plant Physiol. 1961 Jul;36(4):409–414. doi: 10.1104/pp.36.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E., SCHROEDER E. A. The reductive pentose phosphate cycle. II. Specific C-1 phosphatases for fructose 1,6-diphosphate and sedoheptulose 1,7-diphosphate. Arch Biochem Biophys. 1958 Apr;74(2):326–344. doi: 10.1016/0003-9861(58)90004-3. [DOI] [PubMed] [Google Scholar]
- SCOTT D. B. M., COHEN S. S. Enzymatic formation of pentose phosphate from 6-phosphogluconate. J Biol Chem. 1951 Feb;188(2):509–530. [PubMed] [Google Scholar]
- SEGAL S., FOLEY J. B. The metabolic fate of C14 labeled pentoses in man. J Clin Invest. 1959 Feb;38(2):407–413. doi: 10.1172/JCI103815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRERE P., COOPER J. R., TABACHNICK M., RACKER E. The oxidative pentose phosphate cycle. I. Preparation of substrates and enzymes. Arch Biochem Biophys. 1958 Apr;74(2):295–305. doi: 10.1016/0003-9861(58)90001-8. [DOI] [PubMed] [Google Scholar]
- STJERNHOLM R. L., NOBLE E. P. Carbohydrate metabolism in leucocytes. V. The metabolism of five-carbon substrates in polymorphonuclear leucocytes of rabbit. Arch Biochem Biophys. 1963 Feb;100:200–204. doi: 10.1016/0003-9861(63)90062-6. [DOI] [PubMed] [Google Scholar]
- Severin S. E., Stepanova N. G. Izuchenie biosinteza pentozofosfatov v myshtse serdtsa i roli éritrozo-4-fosfata v étom protsesse. Biokhimiia. 1973 May-Jun;38(3):583–588. [PubMed] [Google Scholar]
- Shafrir E., Gutman A., Gorin E., Orevi M. Regulatory aspects in carbohydrate metabolism of adipose tissue: glycolysis, glycogen synthesis, and glyceroneogenesis. Horm Metab Res. 1970;2(Suppl):130–135. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- UTTER M. F. Carbohydrate metabolism. Annu Rev Biochem. 1958;27(3):245–284. doi: 10.1146/annurev.bi.27.070158.001333. [DOI] [PubMed] [Google Scholar]
- VENKATARAMAN R., RACKER E. Mechanism of action of transaldolase. I. Crystalization and properties of yeast enzyme. J Biol Chem. 1961 Jul;236:1876–1882. [PubMed] [Google Scholar]
- VOLK W. A. The enzymatic formation of D-arabinose 5-phosphate from L-arabinose and adenosine triphosphate by Propionibacterium pentosaceum. J Biol Chem. 1959 Aug;234(8):1931–1936. [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- WOOD H. G., KATZ J. The distribution of C14 in the hexose phosphates and the effect of recycling in the pentose cycle. J Biol Chem. 1958 Dec;233(6):1279–1282. [PubMed] [Google Scholar]
- Williams J. F., Clark M. G., Blackmore P. F. The fate of 14C in glucose 6-phosphate synthesized from [1-14C]Ribose 5-phosphate by enzymes of rat liver. Biochem J. 1978 Oct 15;176(1):241–256. doi: 10.1042/bj1760241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Rienits K. G., Schofield P. J., Clark M. G. The pentose phosphate pathway in rabbit liver. Studies on the metabolic sequence and quantitative role of the pentose phosphate cycle by using a system in situ. Biochem J. 1971 Aug;123(5):923–943. doi: 10.1042/bj1230923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. The detection and identification of intermediates of the pentose phosphate cycle and related compounds. J Chromatogr. 1968 Jun 18;35(3):352–361. doi: 10.1016/s0021-9673(01)82396-7. [DOI] [PubMed] [Google Scholar]


