Abstract
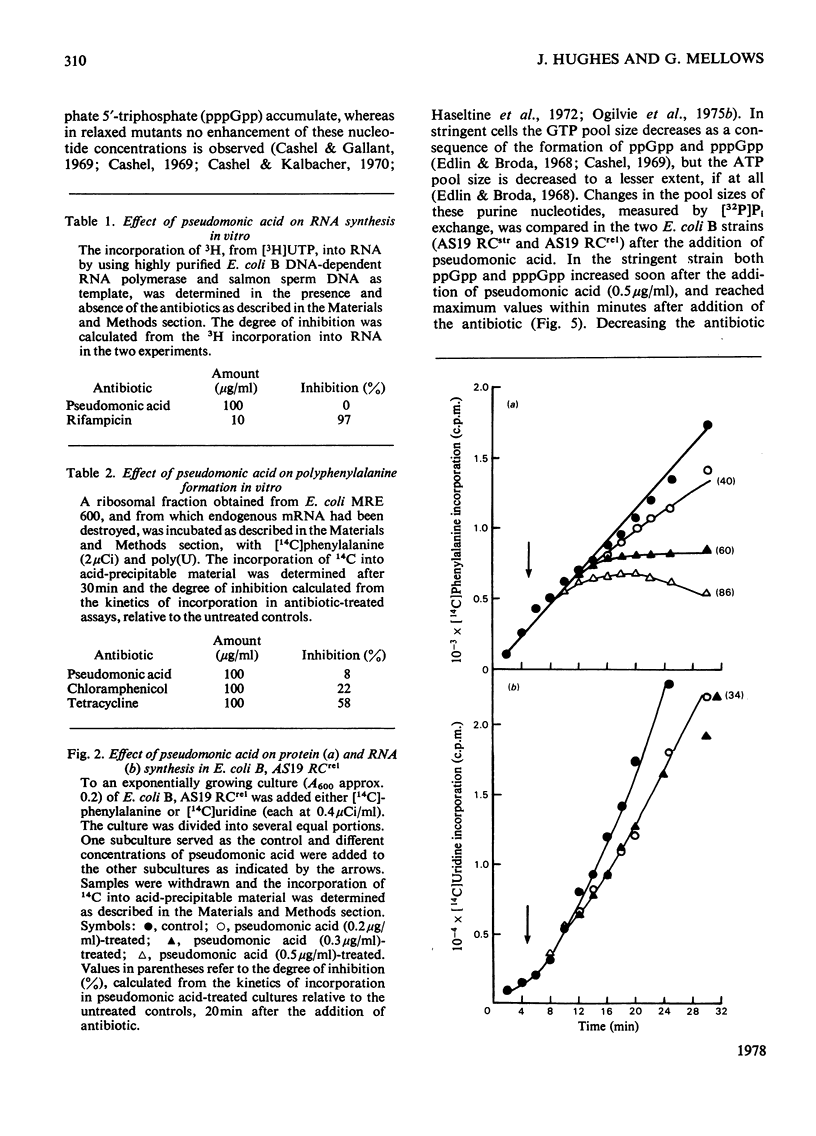
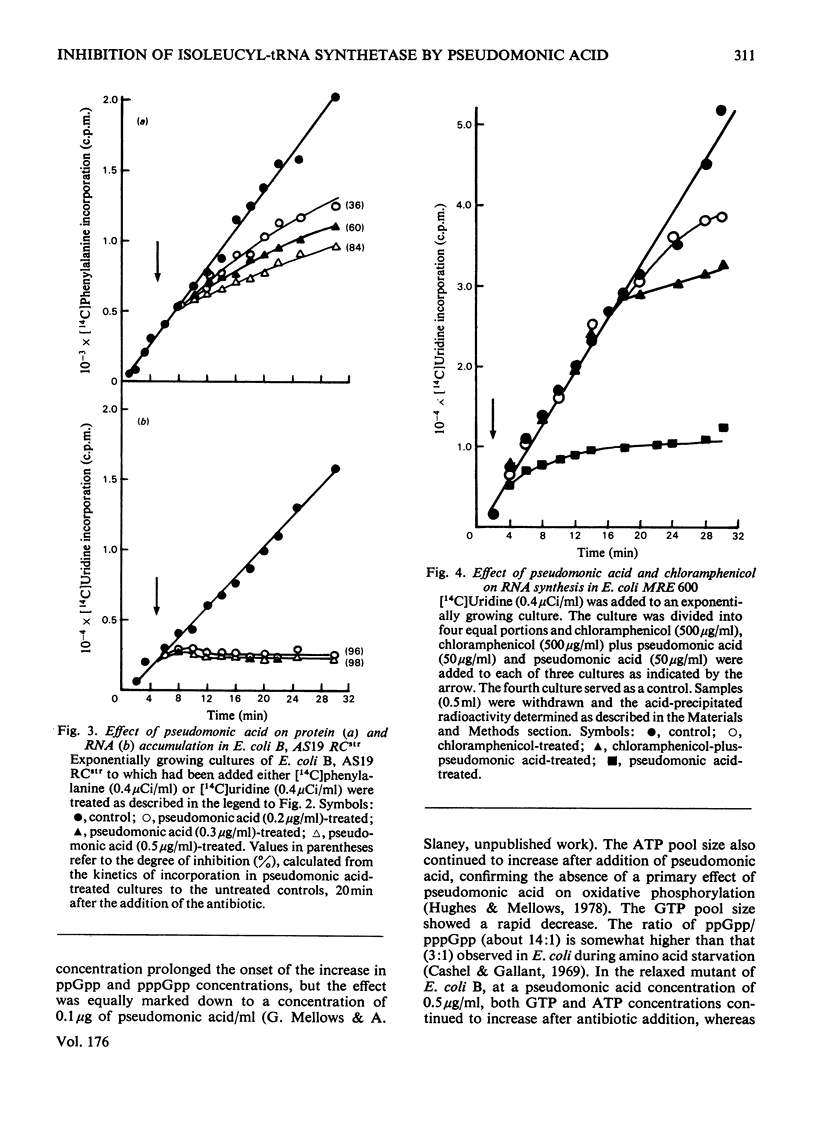
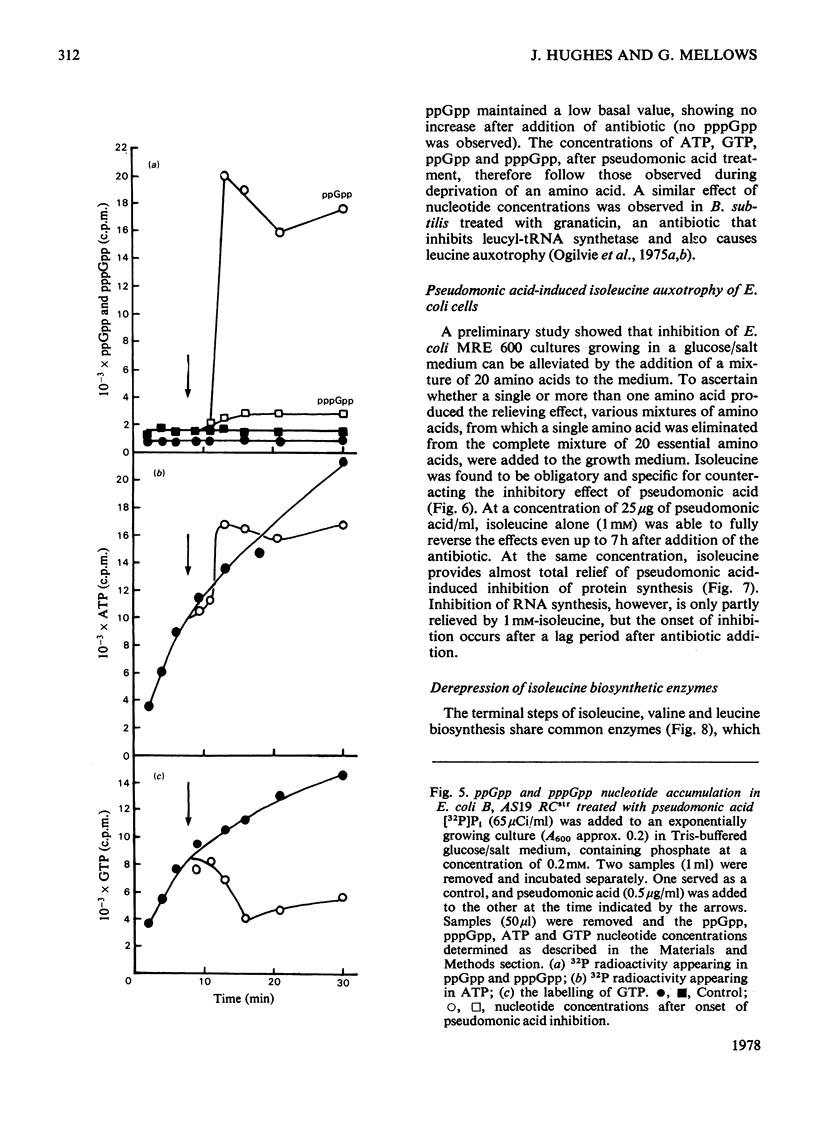
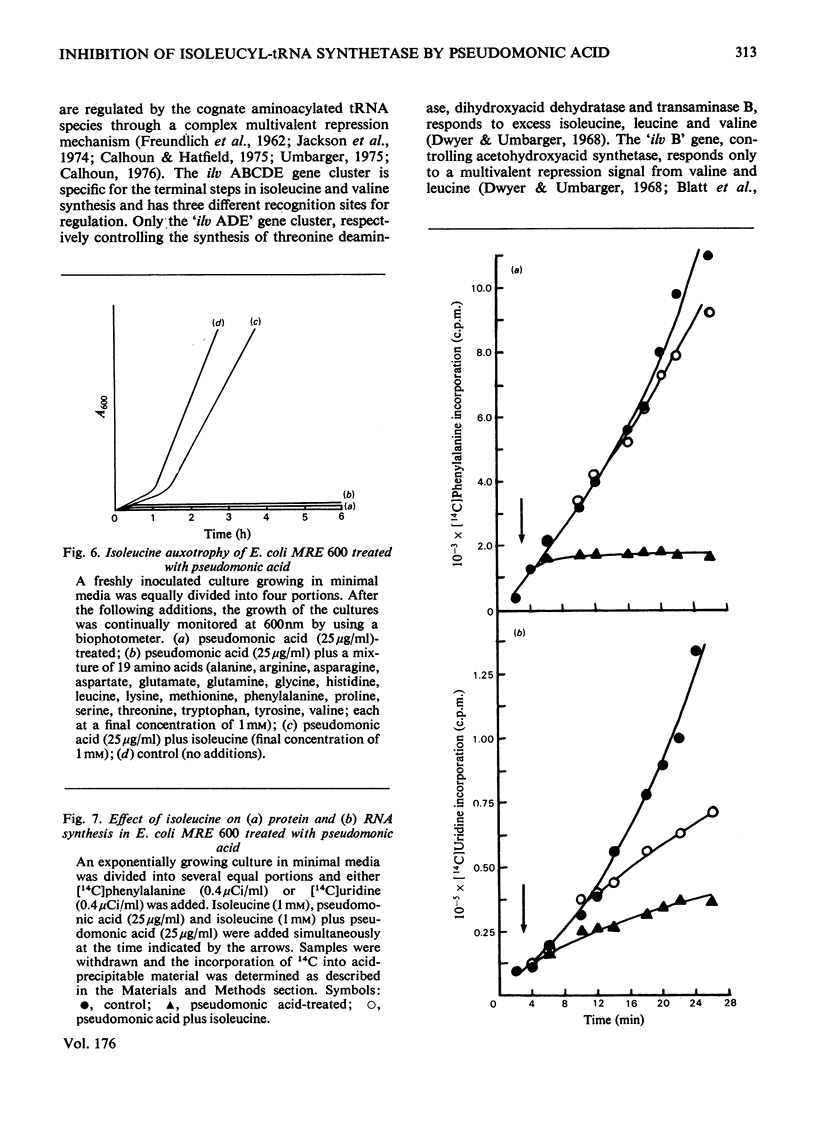
The mode of action of the antibiotic pseudomonic acid has been studied in Escherichia coli. Pseudomonic acid strongly inhibits protein and RNA synthesis in vivo. The antibiotic had no effect on highly purified DNA-dependent RNA polymerase and showed only a weak inhibitory effect on a poly(U)-directed polyphenylalanine-forming ribosomal preparation. Chloramphenicol reversed inhibition of RNA synthesis in vivo. Pseudomonic acid had little effect on RNA synthesis in a regulatory mutant, E. coli B AS19 RCrel, whereas protein synthesis was strongly inhibited. In pseudomonic acid-treated cells, increased concentrations of ppGpp, pppGpp and ATP were observed, but the GTP pool size decreased, suggesting that inhibition of RNA synthesis is a consequence of the stringent control mechanism imposed by pseudomonic acid-induced deprivation of an amino acid. Of the 20 common amino acids, only isoleucine reversed the inhibitory effect in vivo. The antibiotic was found to be a powerful inhibitor of isoleucyl-tRNA synthetase both in vivo and in vitro. Of seven other tRNA synthetases assayed, only a weak inhibitory effect on phenylalanyl-tRNA synthetase was observed; this presumably accounted for the weak effect on polyphenylalanine formation in a ribosomal preparation. Pseudomonic acid also significantly de-repressed threonine deaminase and transaminase B activity, but not dihydroxyacid dehydratase (isoleucine-biosynthetic enzymes) by decreasing the supply of aminoacylated tRNAIle. Pseudomonic acid is the second naturally occurring inhibitor of bacterial isoleucyl-tRNA synthetase to be discovered, furanomycin being the first.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. R., Calvo J. M., Freundlich M. Mutants of Salmonella typhimurium with an altered leucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Apr;106(1):213–220. doi: 10.1128/jb.106.1.213-220.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. T., Jr, Santi D. V. Phenylalanyl transfer ribonucleic acid synthetase from Escherichia coli B. Potent inhibition by analogues of N-benzyl-2-phenylethylamine. J Med Chem. 1976 Nov;19(11):1270–1275. doi: 10.1021/jm00233a002. [DOI] [PubMed] [Google Scholar]
- Arfin S. M., Ratzkin B., Umbarger H. E. The metabolism of valine and isoleucine in Escherichia coli. XVII. The role of induction in the derepression of acetohydroxy acid isomeroreductase. Biochem Biophys Res Commun. 1969 Dec 4;37(6):902–908. doi: 10.1016/0006-291x(69)90216-2. [DOI] [PubMed] [Google Scholar]
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- Blatt J. M., Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XX. Multiple forms of acetohydroxy acid synthetase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):444–450. doi: 10.1016/s0006-291x(72)80071-8. [DOI] [PubMed] [Google Scholar]
- Blatt J. M., Umbarger H. E. On the role of isoleucyl-tRNA synthetase in multivalent repression. Biochem Genet. 1972 Apr;6(2):99–118. doi: 10.1007/BF00486395. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H. Autoregulation of gene expression. Annu Rev Microbiol. 1975;29:275–299. doi: 10.1146/annurev.mi.29.100175.001423. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H. Threonine deaminase from Escherichia coli: feedback-hypersensitive enzyme from a genetic regulatory mutant. J Bacteriol. 1976 Apr;126(1):56–63. doi: 10.1128/jb.126.1.56-63.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Cassio D., Mathien Y. Effect of L-methioninyl adenylate on the level of aminoacylation in vivo of tRNA(Met) from Escherichia coli K12. Nucleic Acids Res. 1974 May;1(5):719–725. doi: 10.1093/nar/1.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dureković A., Flossdorf J., Kula M. R. Isolation and properties of isoleucyl-tRNA synthetase from Escherichia coli MRE 600. Eur J Biochem. 1973 Jul 16;36(2):528–533. doi: 10.1111/j.1432-1033.1973.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flossdorf J., Prätorius H. J., Kula M. R. Influence of side-chain structure of aliphatic amino acids on binding to isoleucyl-tRNA synthetase from Escherichia coli MRE 600. Eur J Biochem. 1976 Jun 15;66(1):147–155. doi: 10.1111/j.1432-1033.1976.tb10435.x. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Isolation and partial characterization of Escherichia coli mutants with altered glycyl transfer ribonucleic acid synthetases. J Bacteriol. 1970 Apr;102(1):193–203. doi: 10.1128/jb.102.1.193-203.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Schlessinger D. Magic spot metabolism in an Escherichia coli mutant temperature sensitive in elongation factor Ts. J Bacteriol. 1974 Mar;117(3):1195–1200. doi: 10.1128/jb.117.3.1195-1200.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould H. J., Loviny T. F., Vasu S. S., Herbert B. N. Biosynthesis of the crystal protein of Bacillus thuringiensis var. tolworth. 2. On the relation of transcriptional and translational events in the growth cycle. Eur J Biochem. 1973 Sep 3;37(3):449–458. doi: 10.1111/j.1432-1033.1973.tb03005.x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler E., Rainey P., Orme A., Bennett E. L., Calvin M. On the active site topography of isoleucyl transfer ribonucleic acid synthetase of Escherichia coli B. Biochemistry. 1973 Mar 13;12(6):1150–1159. doi: 10.1021/bi00730a021. [DOI] [PubMed] [Google Scholar]
- Hughes J., Mellows G. On the mode of action of pseudomonic acid: inhibition of protein synthesis in Staphylococcus aureus. J Antibiot (Tokyo) 1978 Apr;31(4):330–335. doi: 10.7164/antibiotics.31.330. [DOI] [PubMed] [Google Scholar]
- Hütter R., Poralla K., Zachau H. G., Zähner H. Stoffwechselprodukte von Mikroorganismen. 51. Uber die Wirkungsweise von Borrelidin-Hemmung des Threonineinbaus in sRNA. Biochem Z. 1966 Mar 28;344(2):190–196. [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J., Williams L. S., Umbarger H. E. Regulation of synthesis of the branched-chain amino acids and cognate aminoacyl-transfer ribonucleic acid synthetases of Escherichia coli: a common regulatory element. J Bacteriol. 1974 Dec;120(3):1380–1386. doi: 10.1128/jb.120.3.1380-1386.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad I., Röschenthaler R. Inhibition of phenylalanine tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett. 1977 Nov 15;83(2):341–347. doi: 10.1016/0014-5793(77)81037-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Lund E., Kjeldgaard N. O. Metabolism of guanosine tetraphosphate in Escherichia coli. Eur J Biochem. 1972 Jul 24;28(3):316–326. doi: 10.1111/j.1432-1033.1972.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Nass G., Hasenbank R. Effect of Borrelidin on the threonyl-tRNA-synthetase activity and the regulation of threonine-biosynthetic enzymes in Saccharomyces cerivisiae. Mol Gen Genet. 1970;108(1):28–32. doi: 10.1007/BF00343181. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie A., Wiebauer K., Kersten W. Inhibition of leucyl-transfer ribonucleic acid synthetasymol. Biochem J. 1975 Dec;152(3):511–515. doi: 10.1042/bj1520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie A., Wiebauer K., Kersten W. Stringent control of ribonucleic acid synthesis in Bacillus subtilis treated with granaticin. Biochem J. 1975 Dec;152(3):517–522. doi: 10.1042/bj1520517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Lund E., Kjeldgaard N. O. Codon specific, tRNA dependent in vitro synthesis of ppGpp and pppGpp. Nat New Biol. 1973 May 2;243(122):13–15. [PubMed] [Google Scholar]
- Ryan A. M., Borek E. The relaxed control phenomenon. Prog Nucleic Acid Res Mol Biol. 1971;11:193–228. doi: 10.1016/s0079-6603(08)60328-1. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi I. R., Shoukry S. The mode of action of ASK-753 on Bacillus subtilis. J Antibiot (Tokyo) 1976 Mar;29(3):303–308. doi: 10.7164/antibiotics.29.303. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Umbarger H. E. Characterization of fusions between the lac operon and the ilv gene cluster in Escherichia coli: ilvC-lac fusions. J Bacteriol. 1977 Dec;132(3):870–875. doi: 10.1128/jb.132.3.870-875.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa Y., Sokawa J., Kaziro Y. Function of the rel gene in Escherichia coli. Nat New Biol. 1971 Nov 3;234(44):7–10. doi: 10.1038/newbio234007a0. [DOI] [PubMed] [Google Scholar]
- Straus D. S., Ames B. N. Histidyl-transfer ribonucleic acid synthetase mutants requiring a high internal pool of histidine for growth. J Bacteriol. 1973 Jul;115(1):188–197. doi: 10.1128/jb.115.1.188-197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Tamaki M., Watanabe S. Effect of furanomycin on the synthesis of isoleucyl-tRNA. Biochim Biophys Acta. 1969 Nov 19;195(1):244–245. doi: 10.1016/0005-2787(69)90621-2. [DOI] [PubMed] [Google Scholar]
- Werner R. G., Thorpe L. F., Reuter W., Nierhaus K. H. Indolmycin inhibits prokaryotic tryptophanyl-tRNA ligase. Eur J Biochem. 1976 Sep;68(1):1–3. doi: 10.1111/j.1432-1033.1976.tb10758.x. [DOI] [PubMed] [Google Scholar]


