Abstract
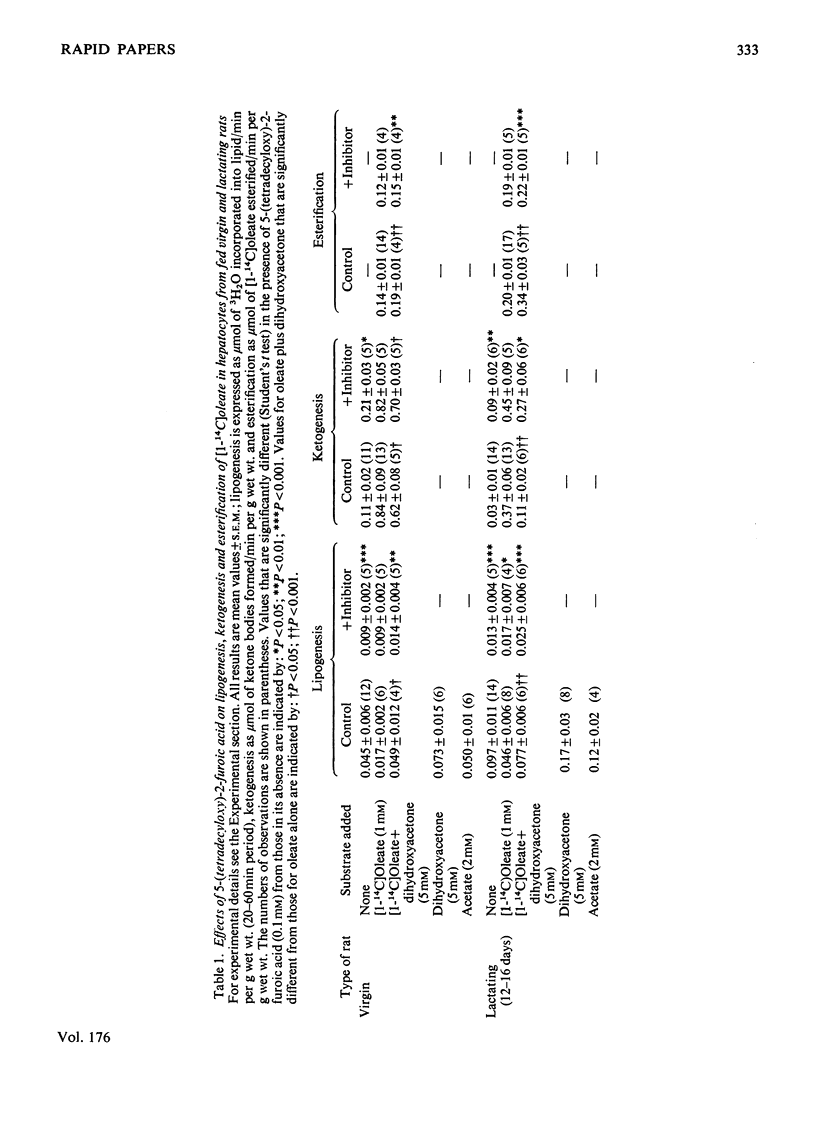
Lipogenesis is increased in hepatocytes from fed lactating rats compared with virgin rats. Inhibition of lipogenesis with 5-(tetradecyloxy)-2-furoic acid resulted in increased ketogenesis from endogenous substrate, but not from oleate. Dihydroxyacetone increased ketogenesis from endogenous substrate, but not from oleate. Dihydroxyacetone increased lipogenesis and esterification of [1--14C]oleate and decreased ketogenesis; these changes were reversed by the inhibitor. The reciprocal relationship between lipogenesis and ketogenesis in hepatocytes from fed rats may be due to alterations in [malonyl-CoA] [McGarry, Mannaerts & Foster (1977) J. Clin. Invest. 60, 265--270; Cook, King & Veech (1978) J. Biol. Chem. 253, 2529--2531], but this mechanism is not considered to be sufficient to explain the increased ketogenesis in starvation completely.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A., King M. T., Veech R. L. Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. J Biol Chem. 1978 Apr 25;253(8):2529–2531. [PubMed] [Google Scholar]
- Cook G. A., Nielsen R. C., Hawkins R. A., Mehlman M. A., Lakshmanan M. R., Veech R. L. Effect of glucagon on hepatic malonyl coenzyme A concentration and on lipid synthesis. J Biol Chem. 1977 Jun 25;252(12):4421–4424. [PubMed] [Google Scholar]
- FELL B. F., SMITH K. A., CAMPBELL R. M. Hypertrophic and hyperplastic changes in the alimentary canal of the lactating rat. J Pathol Bacteriol. 1963 Jan;85:179–188. doi: 10.1002/path.1700850117. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Harris R. A. Studies on the inhibition of hepatic lipogenesis by N-6,O-2'-dibutyryl adenosine 3',5'-monophosphate. Arch Biochem Biophys. 1975 Jul;169(1):168–180. doi: 10.1016/0003-9861(75)90330-6. [DOI] [PubMed] [Google Scholar]
- Kariya T., Wille L. J. Inhibition of fatty acid synthesis by RMI 14,514 (5-tetradecyloxy-2-furoic acid). Biochem Biophys Res Commun. 1978 Feb 28;80(4):1022–1024. doi: 10.1016/0006-291x(78)91347-5. [DOI] [PubMed] [Google Scholar]
- Mapes J. P. Inhibition of lipogenesis by halothane in isolated rat liver cells. Biochem J. 1977 Jan 15;162(1):47–50. doi: 10.1042/bj1620047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Topping D. L. Regulation of hepatic lipogenesis by plasma free fatty acids: simultaneous studies on lipoprotein secretion, cholesterol synthesis, ketogenesis and gluconeogenesis. Biochem J. 1974 Apr;140(1):111–114. doi: 10.1042/bj1400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek E., Cook G. A., Cornell N. W. Inhibition by 5-(tetradecyloxy)-2-furoic acid of fatty acid and cholesterol synthesis in isolated rat hepatocytes. Lipids. 1977 Oct;12(10):814–818. doi: 10.1007/BF02533270. [DOI] [PubMed] [Google Scholar]
- Ribereau-Gayon G. Inhibition of mitochondrial tricarboxylate anion translocation and of liver fatty acid synthesis by a new hypolipidemic agent. FEBS Lett. 1976 Mar 1;62(3):309–312. doi: 10.1016/0014-5793(76)80082-8. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Control of glucose metabolism in isolated acini of the lactating mammary gland of the rat. The ability of glycerol to mimic some of the effects of insulin. Biochem J. 1977 Dec 15;168(3):465–474. doi: 10.1042/bj1680465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. W. The effects of pregnancy, lactation and involution on the metabolism of glucose and acetate by rat liver tissue. J Dairy Res. 1973 Oct;40(3):339–351. doi: 10.1017/s0022029900014710. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., McKeown S. R., Ilic V. Interactions of glucose, acetoacetate and insulin in mammary-gland slices of lactating rats. Biochem J. 1975 Aug;150(2):145–152. doi: 10.1042/bj1500145. [DOI] [PMC free article] [PubMed] [Google Scholar]



