Abstract
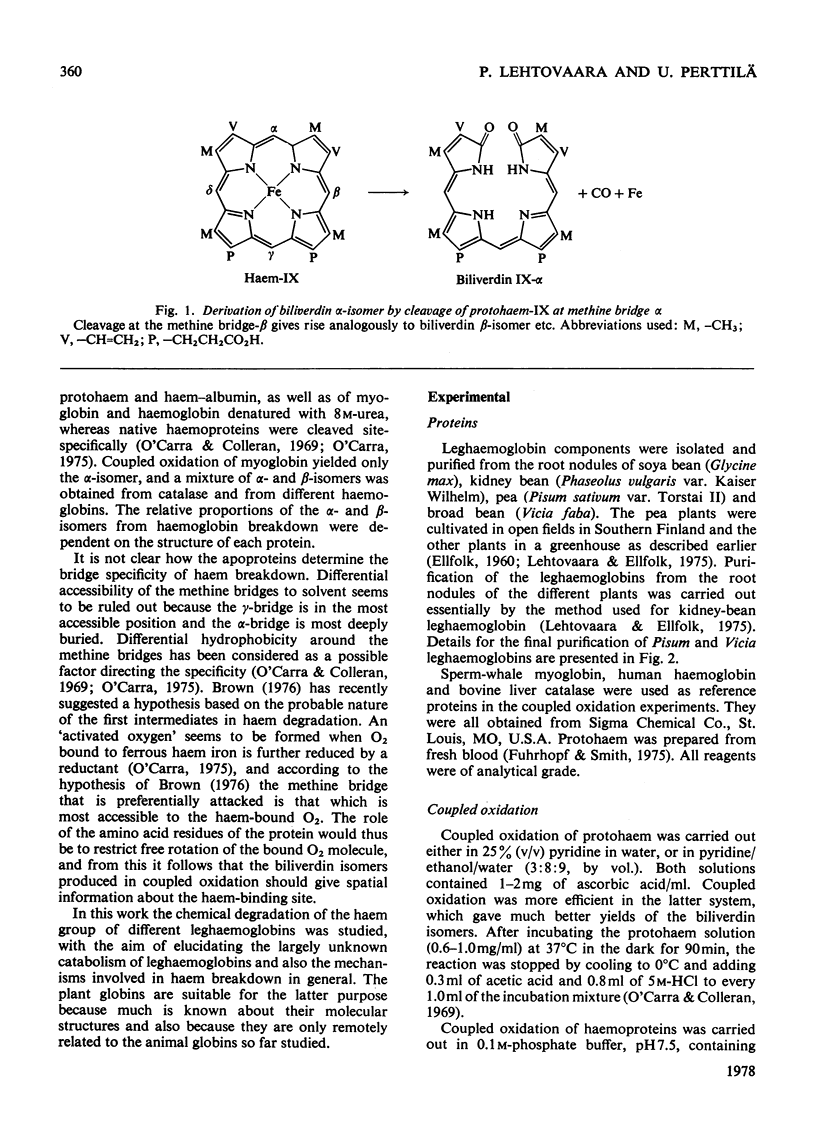
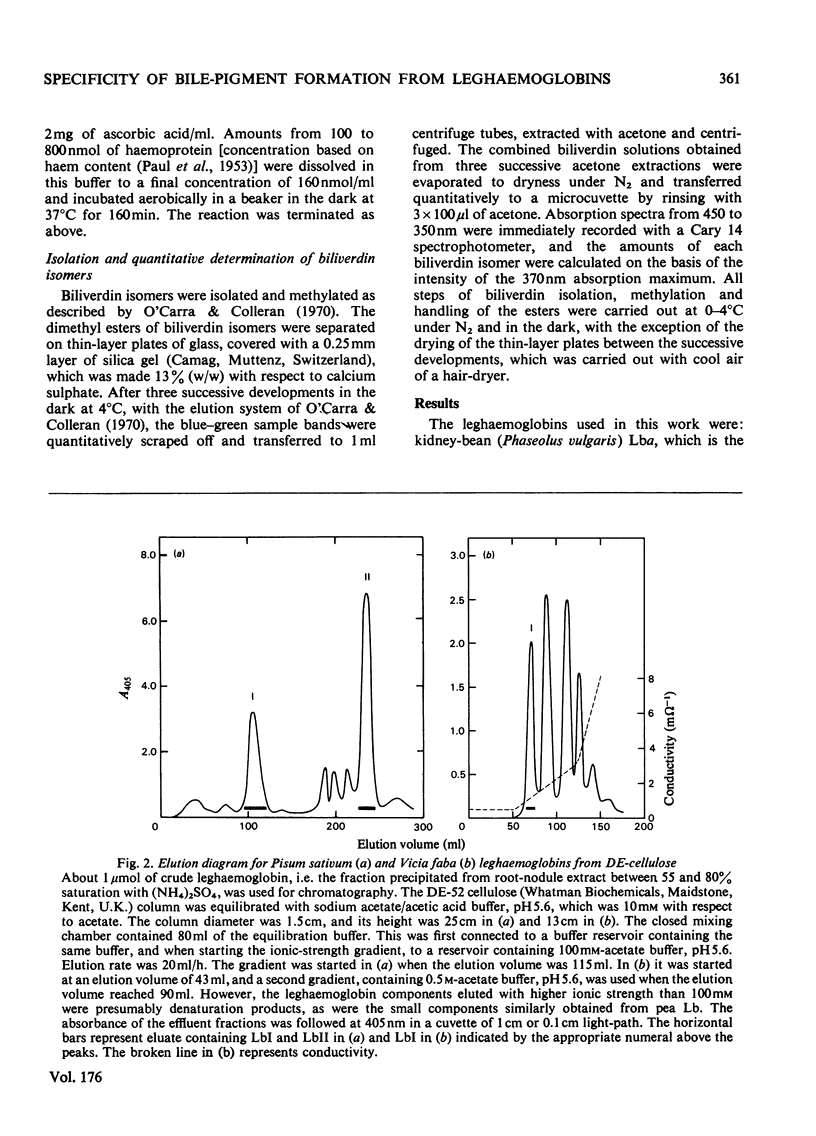
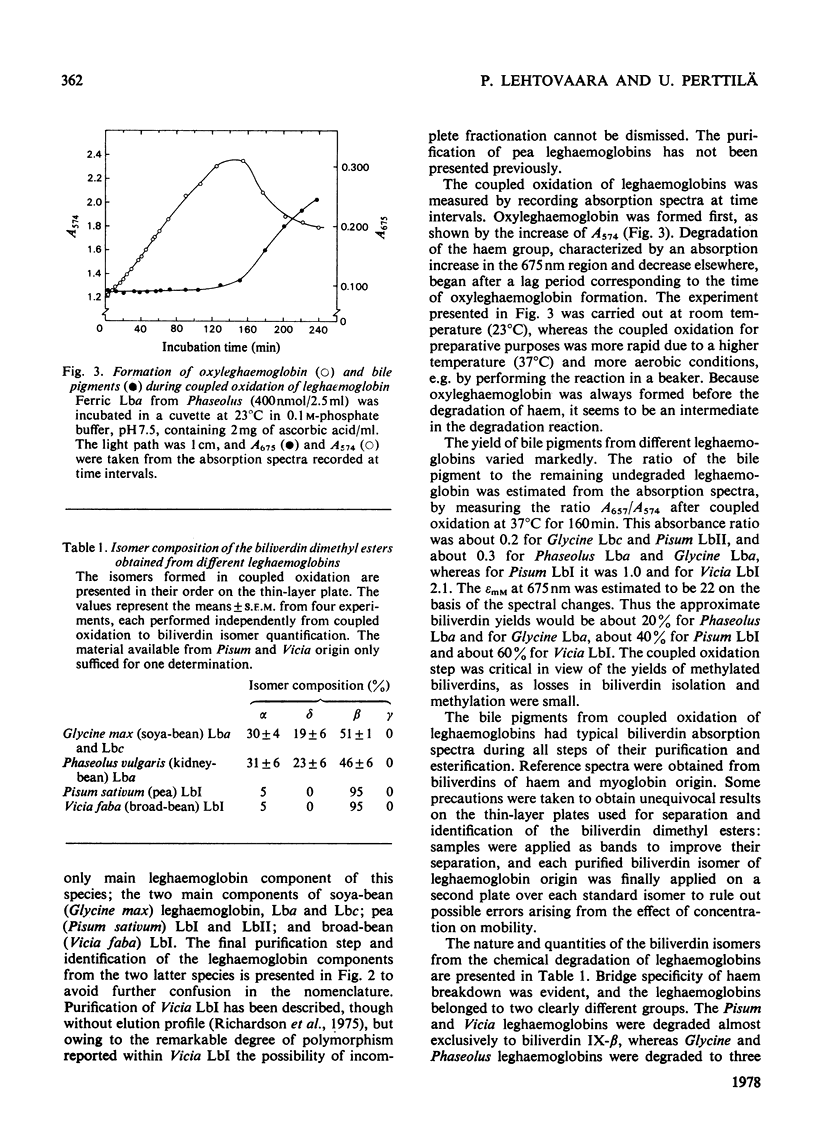
The coupled oxidation of leghaemoglobins with O2 and ascorbate yielded oxyleghaemoglobin in the first reaction step, and the second step was the degradation of haem characterized by an A675 increase. Leghaemoglobins were degraded to biliverdin isomers specifically, depending on the structure of the protein. The main leghaemoglobin components of Glycine (soya bean) and Phaseolus (kidney bean) were degraded to biliverdin mixtures containing about 50% of the β-form, about 30% of the α-form and about 20% of the δ-isomer, whereas the leghaemoglobin I components of Vicia (broad bean) and Pisum (pea) were degraded almost exclusively to the β-isomer, with traces of the α-isomer. The amino acid sequences of Glycine and Phaseolus leghaemoglobins resemble each other, as do those of Vicia and Pisum. The site specificity of bile-pigment formation from leghaemoglobins can be tentatively explained by specific differences in the amino acid sequences at those regions of the polypeptide chain that are in the vicinity of the appropriate methine bridges. The ligand-binding site in different leghaemoglobins may be outlined on the basis of the present results, supposing that the haem is degraded when a reduction product of haem-bound O2 reacts with a methine bridge of the haem, and that the bridge specificity is regulated by hindering amino acid residues that determine the location of the bound O2. The residue phenylalanine-CD1 appears to be further away from the haem plane or in a markedly more flexible position in leghaemoglobins than in mammalian globins. The haem-bound oxygen atom B, in Fe–O(A)–O(B), seems to be free to rotate in all directions except that of the γ-bridge in Glycine and Phaseolus leghaemoglobins, but its position in Vicia and Pisum leghaemoglobin I might be restricted to the direction of the β-methine bridge.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Docherty J. C., Bradley T. B. Bile-pigment isomers from degradation of haemoglobin M Iwate [proceedings]. Biochem Soc Trans. 1977;5(4):1020–1022. doi: 10.1042/bst0051020. [DOI] [PubMed] [Google Scholar]
- Brown S. B. Stereospecific haem cleavage. A model for the formation of bile-pigment isomers in vivo and in vitro. Biochem J. 1976 Oct 1;159(1):23–27. doi: 10.1042/bj1590023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellfolk N., Sievers G. Correction of the amino acid sequence of soybean leghemoglobin alpha. Acta Chem Scand B. 1974;28(10):1245–1246. doi: 10.3891/acta.chem.scand.28b-1245. [DOI] [PubMed] [Google Scholar]
- Ellfolk N., Sievers G. The primary structure of soybean leghemoglobin. Acta Chem Scand. 1971;25(9):3532–3534. doi: 10.3891/acta.chem.scand.25-3532. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Love W. E., Karle J. Crystal structure analysis of sea lamprey hemoglobin at 2 angstrom resolution. J Mol Biol. 1973 Mar 5;74(3):331–361. doi: 10.1016/0022-2836(73)90377-x. [DOI] [PubMed] [Google Scholar]
- Huber R., Epp O., Steigemann W., Formanek H. The atomic structure of erythrocruorin in the light of the chemical sequence and its comparison with myoglobin. Eur J Biochem. 1971 Mar 1;19(1):42–50. doi: 10.1111/j.1432-1033.1971.tb01285.x. [DOI] [PubMed] [Google Scholar]
- KENDREW J. C., WATSON H. C., STRANDBERG B. E., DICKERSON R. E., PHILLIPS D. C., SHORE V. C. The amino-acid sequence x-ray methods, and its correlation with chemical data. Nature. 1961 May 20;190:666–670. doi: 10.1038/190666a0. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Ellfolk N. The amino-acid sequence of leghemoglobin component a from Phaseolus vulgaris (kidney bean). Eur J Biochem. 1975 Jun;54(2):577–584. doi: 10.1111/j.1432-1033.1975.tb04170.x. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Ellfolk N. The primary structure of kidney bean leghemoglobin. FEBS Lett. 1974 Jul 15;43(2):239–240. doi: 10.1016/0014-5793(74)81009-4. [DOI] [PubMed] [Google Scholar]
- Lemberg R. Transformation of haemins into bile pigments. Biochem J. 1935 Jun;29(6):1322–1336. doi: 10.1042/bj0291322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padlan E. A., Love W. E. Three-dimensional structure of hemoglobin from the polychaete annelid, Glycera dibranchiata, at 2.5 A resolution. J Biol Chem. 1974 Jul 10;249(13):4067–4078. [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Richardson M., Dilworth M. J., Scawen M. D. The amino acid sequence of leghaemoglobin I from root nodules of broad bean (Vicia faba L.). FEBS Lett. 1975 Mar 1;51(1):33–37. doi: 10.1016/0014-5793(75)80849-0. [DOI] [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Vainshtein B. K., Harutyunyan E. H., Kuranova I. P., Borisov V. V., Sosfenov N. I., Pavlovsky A. G., Grebenko A. I., Konareva N. V. Structure of leghaemoglobin from lupin root nodules at 5 angstrom resolution. Nature. 1975 Mar 13;254(5496):163–164. doi: 10.1038/254163a0. [DOI] [PubMed] [Google Scholar]


