Abstract
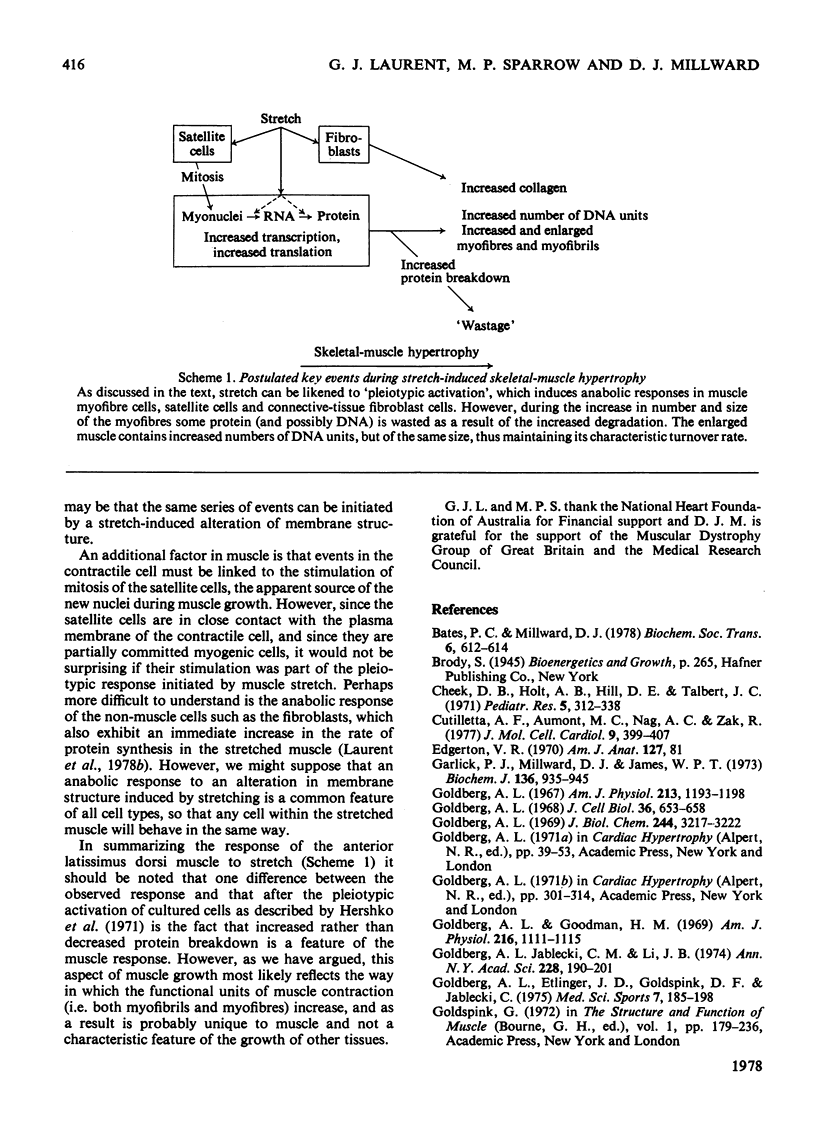
Measurements were made of the growth and of the changes in rates of protein turnover in the anterior latissimus dorsi muscle of the adult fowl in response to the attachment of a weight to one wing. Over 58 days there was a 140% increase in the protein content with similar increases in the RNA and DNA contents. The fractional rate of protein synthesis, measured by the continuous-infusion technique using [14C]proline, increased markedly during hypertrophy. This increase was mediated initially (after 1 day) by an increase in the RNA activity but at all other times reflected the higher RNA content. The rate of protein degradation, calculated from the difference between the synthesis and growth rates, appeared to increase and remain elevated for at least 4 weeks. At no time was there any suggestion of a fall in the rate of degradation. The following events are discussed as central to the changes that occur during skeletal-muscle hypertrophy. 1. Nuclear proliferation is necessary to maintain the characteristic synthesis rate because of the inability of existing nuclei to 'manage' increased protein synthesis for more than a limited period. 2. The increased protein breakdown during hypertrophy is consistent with the known over-production of a new muscle fibres and may indicate some 'wastage' during the growth. Such wastage may also be associated with myofibrillar proliferation. 3. Muscle stretch must be recognized as the major activator of growth and as such can be compared with the 'pleiotypic activators' that have been described for cells in culture.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates P. C., Millward D. J. Changes in the relative rates of protein synthesis and breakdown during muscle growth and atrophy [proceedings]. Biochem Soc Trans. 1978;6(3):612–614. doi: 10.1042/bst0060612. [DOI] [PubMed] [Google Scholar]
- Cutilletta A. F., Aumont M., Nag A., Zak R. Separation of muscle and non-muscle cells from adult rat myocardium: an application to the study of RNA polymerase. J Mol Cell Cardiol. 1977 May;9(5):399–407. doi: 10.1016/s0022-2828(77)80006-0. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R. Morphology and histochemistry of the soleus muscle from normal and exercised rats. Am J Anat. 1970 Jan;127(1):81–87. doi: 10.1002/aja.1001270107. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Etlinger J. D., Goldspink D. F., Jablecki C. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports. 1975 Fall;7(3):185–198. [PubMed] [Google Scholar]
- Goldberg A. L., Goodman H. M. Amino acid transport during work-induced growth of skeletal muscle. Am J Physiol. 1969 May;216(5):1111–1115. doi: 10.1152/ajplegacy.1969.216.5.1111. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Jablecki C., Li J. B. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. Effects of use and disuse on amino acid transport and protein turnover in muscle. Ann N Y Acad Sci. 1974 Mar 22;228(0):190–201. doi: 10.1111/j.1749-6632.1974.tb20510.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol. 1968 Mar;36(3):653–658. doi: 10.1083/jcb.36.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969 Jun 25;244(12):3217–3222. [PubMed] [Google Scholar]
- Goldberg A. L. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967 Nov;213(5):1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- Grimble G. K., Millward D. J. The measurement of ribosomal ribonucleic acid synthesis in rat liver and skeletal muscle in vivo [proceedings]. Biochem Soc Trans. 1977;5(4):913–916. doi: 10.1042/bst0050913. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Erroneous interpretations which may result from application of the "myofibrillar ATPase" histochemical procedure to developing muscle. Exp Neurol. 1972 Mar;34(3):465–475. doi: 10.1016/0014-4886(72)90042-8. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The significance of longitudinal fibre division in skeletal muscle. J Neurol Sci. 1972;15(1):27–33. doi: 10.1016/0022-510x(72)90119-0. [DOI] [PubMed] [Google Scholar]
- Hamosch M., Lesch M., Baron J., Kaufman S. Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science. 1967 Aug 25;157(3791):935–937. doi: 10.1126/science.157.3791.935. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hirsch C. A., Cox M. A., van Venrooij W. J., Henshaw E. C. The ribosome cycle in mammalian protein synthesis. II. Association of the native smaller ribosomal subunit with protein factors. J Biol Chem. 1973 Jun 25;248(12):4377–4385. [PubMed] [Google Scholar]
- Hubbard R. W., Ianuzzo C. D., Mathew W. T., Linduska J. D. Compensatory adaptations of skeletal muscle composition to a long-term functional overload. Growth. 1975 Mar;39(1):85–93. [PubMed] [Google Scholar]
- Jablecki C. K., Heuser J. E., Kaufman S. Autoradiographic localization of new RNA synthesis in hypertrophying skeletal muscle. J Cell Biol. 1973 Jun;57(3):743–759. doi: 10.1083/jcb.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Jefferson L. S., Rannels D. E., Munger B. L., Morgan H. E. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc. 1974 Apr;33(4):1098–1104. [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl (Gallus domesticus). Rates of protein synthesis in fast and slow skeletal, cardiac and smooth muscle of the adult fowl. Biochem J. 1978 Nov 15;176(2):393–401. doi: 10.1042/bj1760393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl. Collagen content and turnover in cardiac and skeletal muscles of the adult fowl and the changes during stretch-induced growth. Biochem J. 1978 Nov 15;176(2):419–427. doi: 10.1042/bj1760419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P. Changes in RNA, DNA and protein content and the rates of protein synthesis and degradation during hypertrophy of the anterior latissimus dorsi muscle of the adult fowl (Gallus domesticus). Growth. 1977 Dec;41(4):249–262. [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Stewart R. J., Nnanyelugo D. O., Waterlow J. C. Skeletal-muscle growth and protein turnover. Biochem J. 1975 Aug;150(2):235–243. doi: 10.1042/bj1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J. Protein turnover in skeletal muscle. II. The effect of starvation and a protein-free diet on the synthesis and catabolism of skeletal muscle proteins in comparison to liver. Clin Sci. 1970 Nov;39(5):591–603. doi: 10.1042/cs0390591. [DOI] [PubMed] [Google Scholar]
- Morton D. J., Rowe R. W. Ultrastructural modifications of mitochondria of rat soleus muscles surgically induced to hypertrophy. Protoplasma. 1974;81(4):335–348. doi: 10.1007/BF01281047. [DOI] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970 Feb;44(2):459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971 Aug;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Narahara H. T., Holloszy J. O. The actions of insulin, trypsin, and electrical stimulation on amino acid transport in muscle. J Biol Chem. 1974 Sep 10;249(17):5435–5443. [PubMed] [Google Scholar]
- Reitsma W. Some structural changes in skeletal muscles of the rat after intensive training. Acta Morphol Neerl Scand. 1970;7(3):229–245. [PubMed] [Google Scholar]
- Schiaffino S., Bormioli S. P., Aloisi M. Cell proliferation in rat skeletal muscle during early stages of compensatory hypertrophy. Virchows Arch B Cell Pathol. 1972;11(3):268–273. doi: 10.1007/BF02889406. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Kaufman S. Enhanced RNA polymerase activity in skeletal muscle undergoing hypertrophy. Arch Biochem Biophys. 1970 Apr;137(2):469–476. doi: 10.1016/0003-9861(70)90464-9. [DOI] [PubMed] [Google Scholar]
- Sola O. M., Christensen D. L., Martin A. W. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol. 1973 Oct;41(1):76–100. doi: 10.1016/0014-4886(73)90182-9. [DOI] [PubMed] [Google Scholar]
- Turner L. V., Garlick P. J. The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta. 1974 Apr 27;349(1):109–113. doi: 10.1016/0005-2787(74)90013-6. [DOI] [PubMed] [Google Scholar]
- Young V. R. Regulation of protein synthesis and skeletal muscle growth. J Anim Sci. 1974 May;38(5):1054–1070. doi: 10.2527/jas1974.3851054x. [DOI] [PubMed] [Google Scholar]


