Abstract
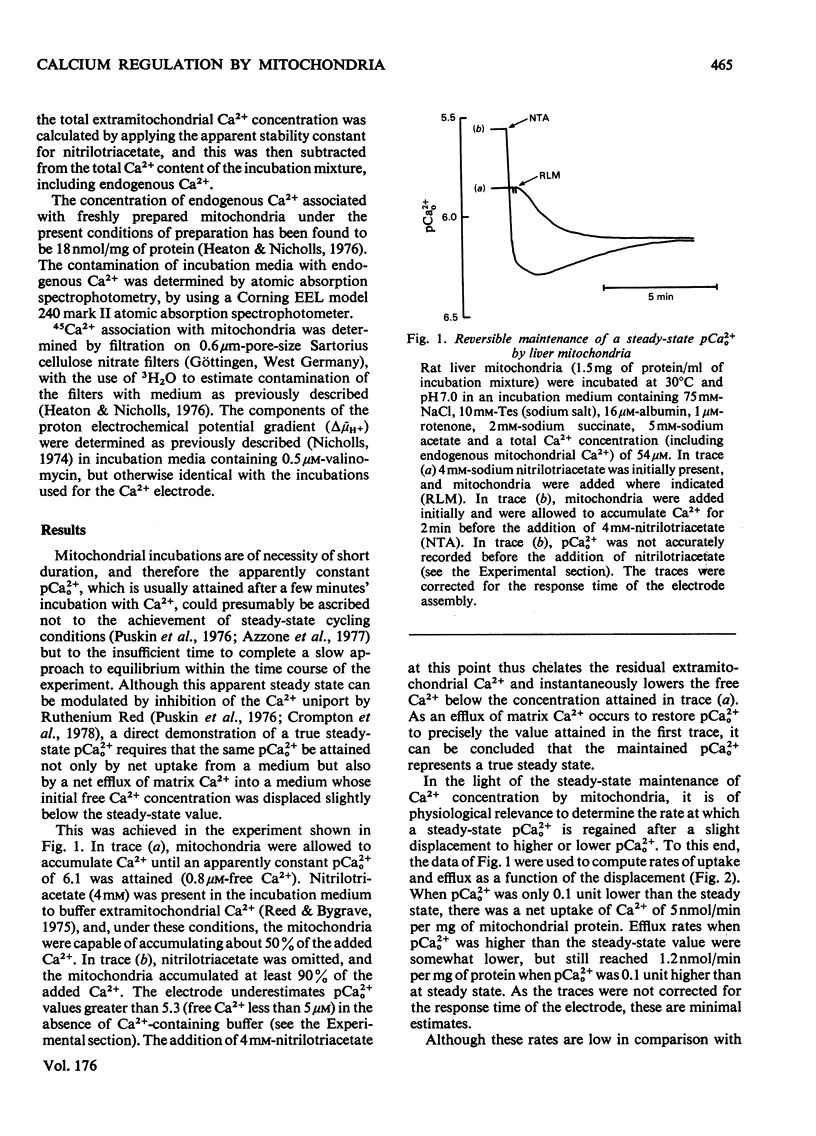
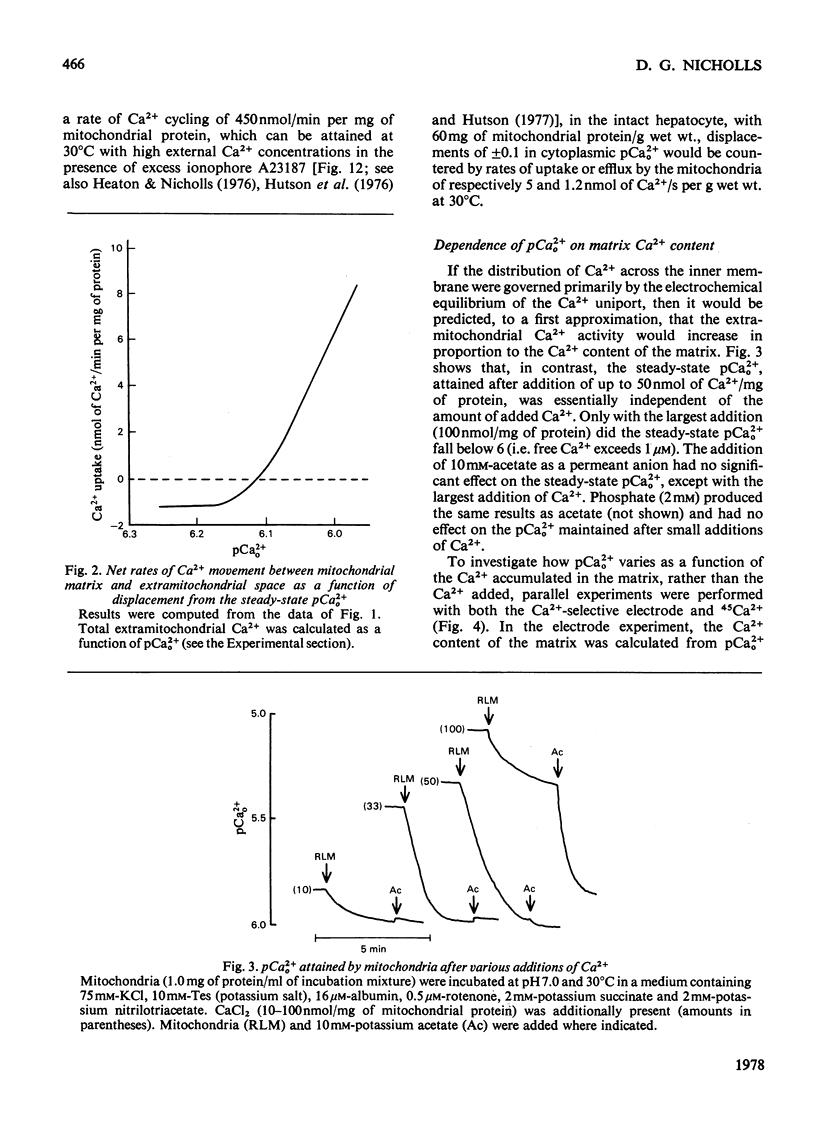
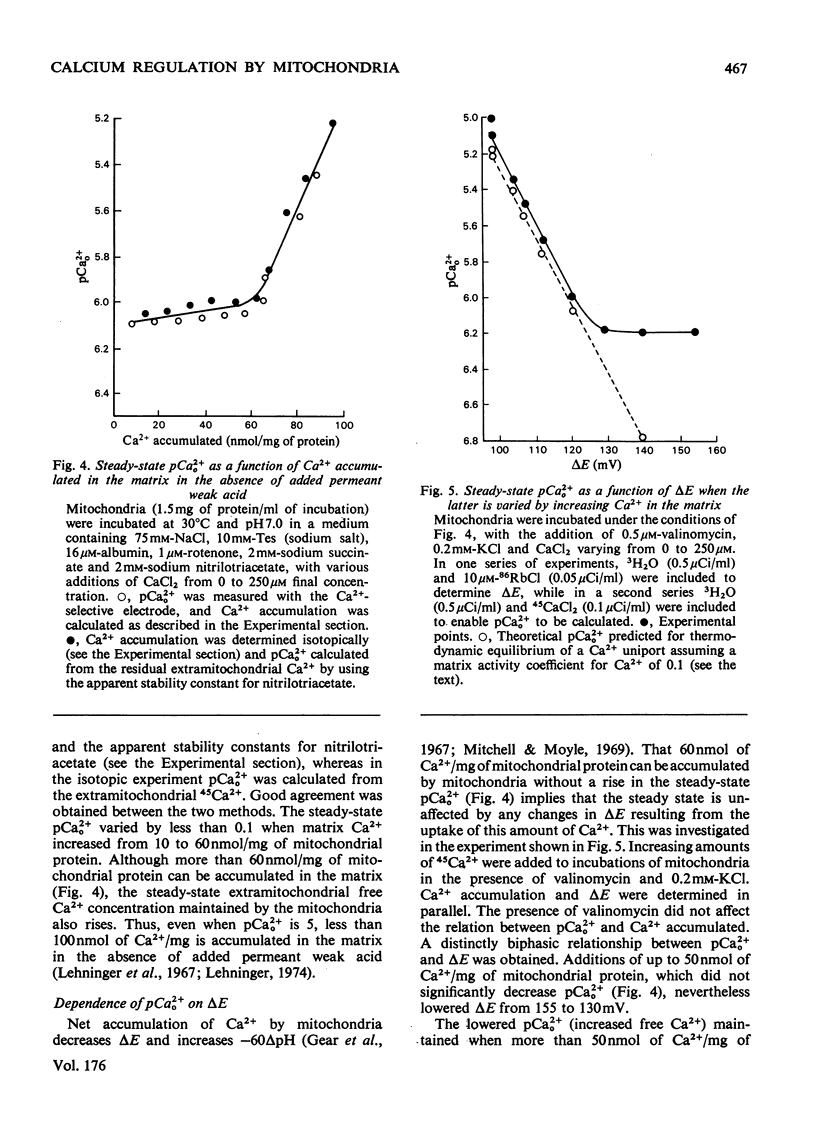
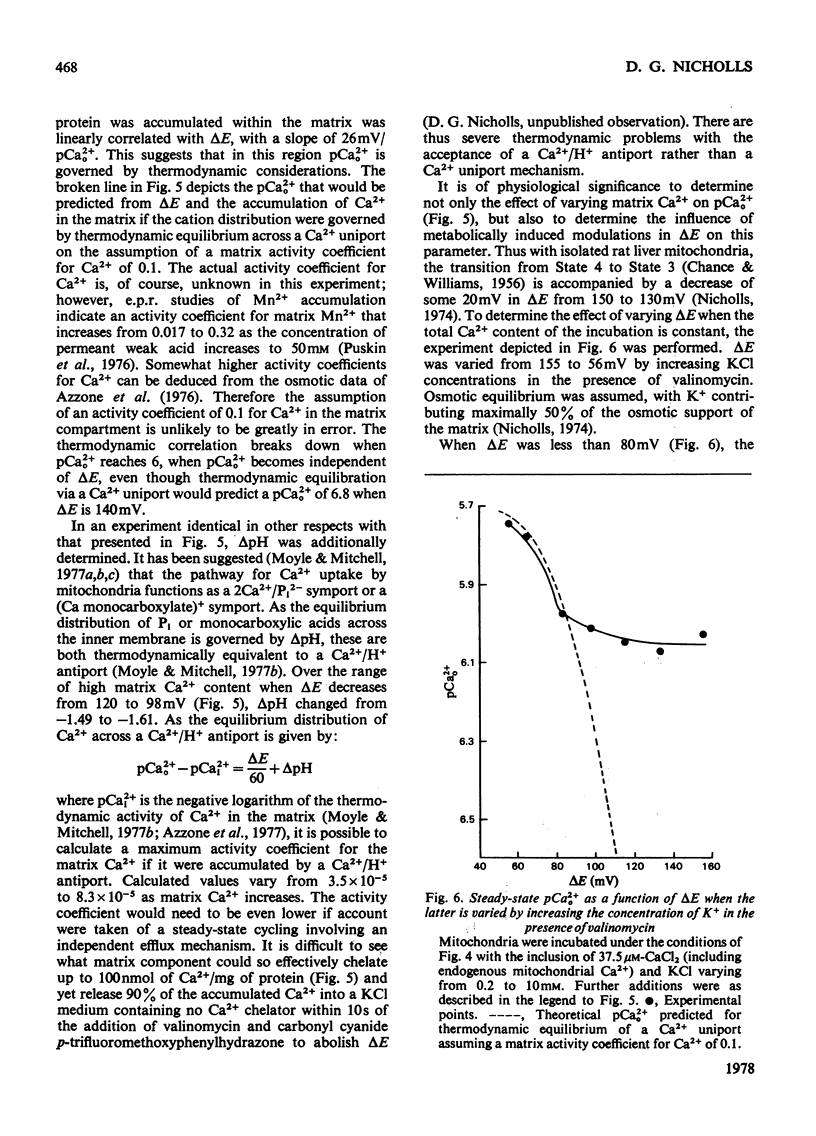
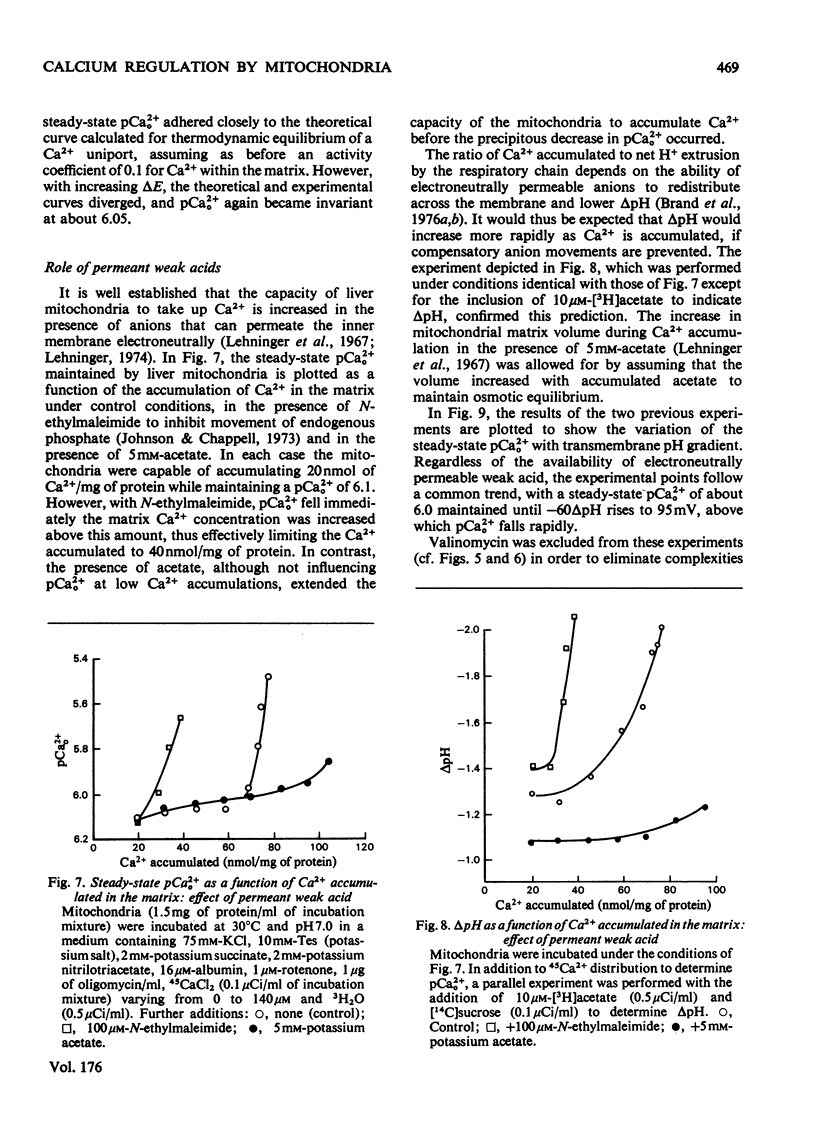
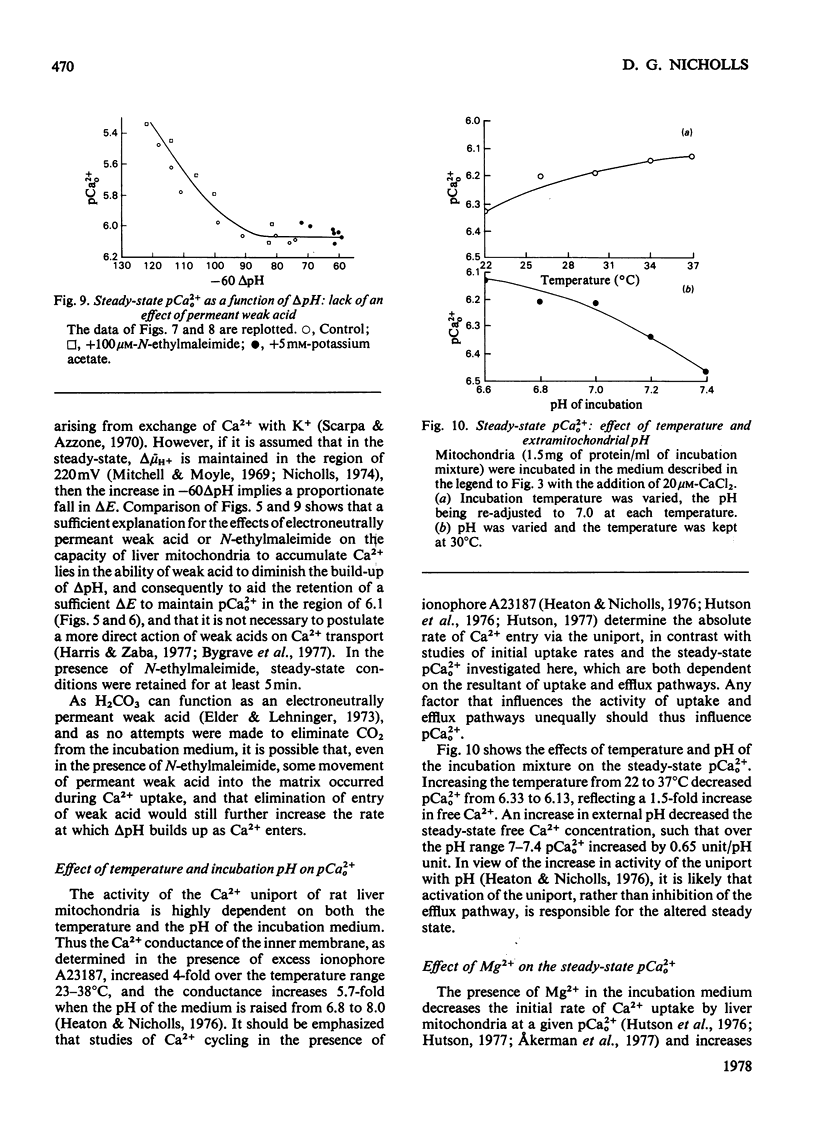
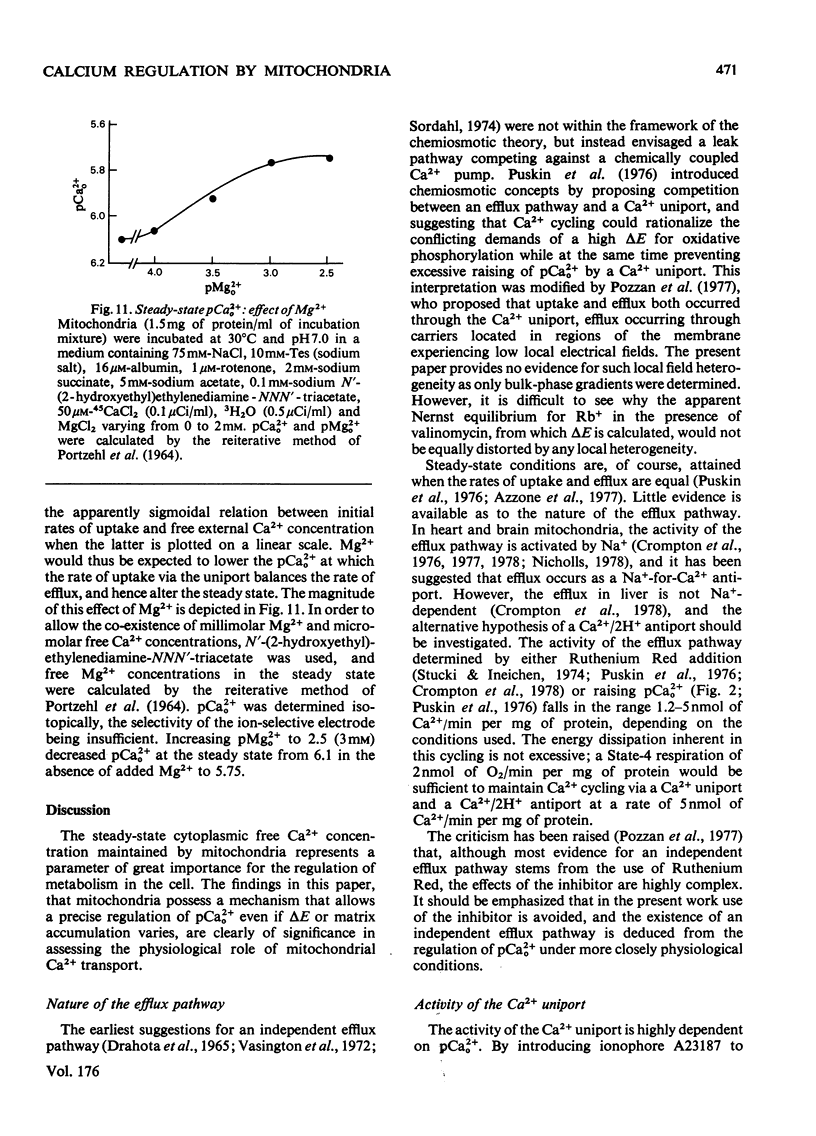
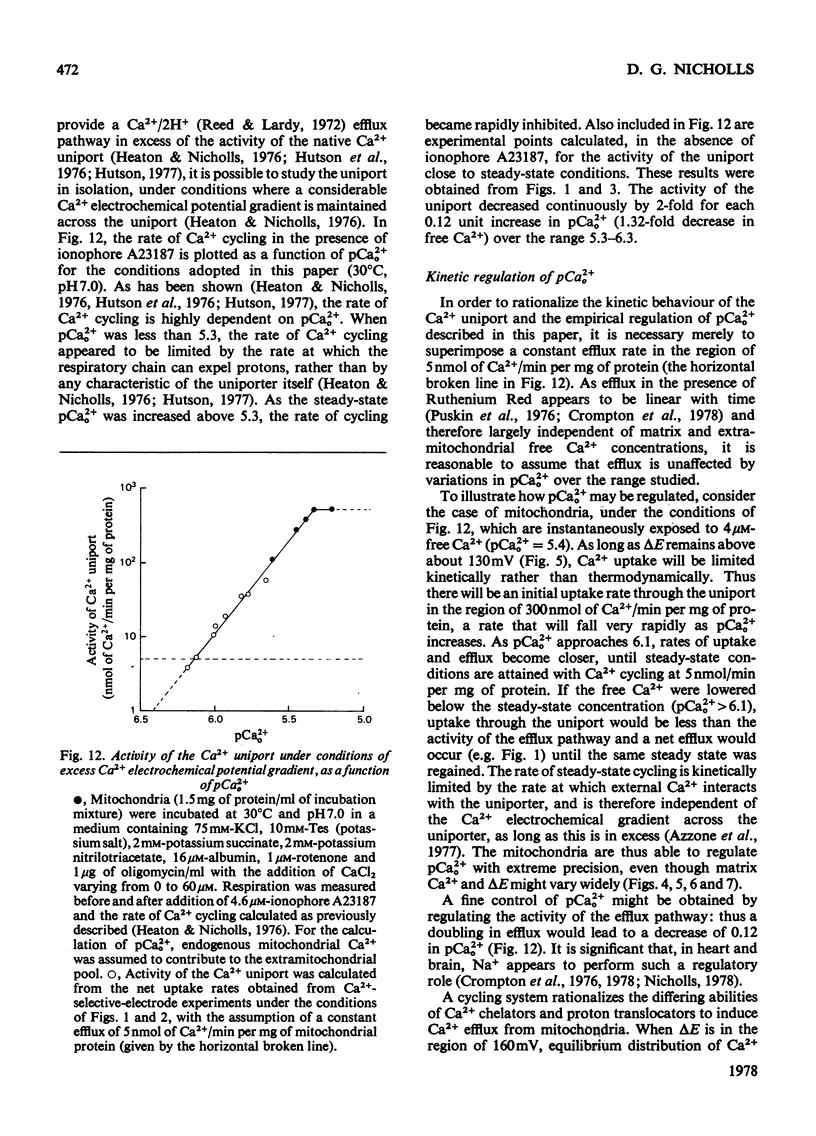
The mechanism whereby rat liver mitochondria regulate the extramitochondrial concentration of free Ca2+ was investigated. At 30°C and pH7.0, mitochondria can maintain a steady-state pCa2+0 (the negative logarithm of the free extramitochondrial Ca2+ concentration) of 6.1 (0.8μm). This represents a true steady state, as slight displacements in pCa2+0 away from 6.1 result in net Ca2+ uptake or efflux in order to restore pCa2+0 to its original value. In the absence of added permeant weak acid, the steady-state pCa2+0 is virtually independent of the Ca2+ accumulated in the matrix until 60nmol of Ca2+/mg of protein has been taken up. The steady-state pCa2+0 is also independent of the membrane potential, as long as the latter parameter is above a critical value. When the membrane potential is below this value, pCa2+0 is variable and appears to be governed by thermodynamic equilibration of Ca2+ across a Ca2+ uniport. Permeant weak acids increase, and N-ethylmaleimide decreases, the capacity of mitochondria to buffer pCa2+0 in the region of 6 (1μm-free Ca2+) while accumulating Ca2+. Permeant acids delay the build-up of the transmembrane pH gradient as Ca2+ is accumulated, and consequently delay the fall in membrane potential to values insufficient to maintain a pCa2+0 of 6. The steady-state pCa2+0 is affected by temperature, incubation pH and Mg2+. The activity of the Ca2+ uniport, rather than that of the respiratory chain, is rate-limiting when pCa2+0 is greater than 5.3 (free Ca2+ less than 5μm). When the Ca2+ electrochemical gradient is in excess, the activity of the uniport decreases by 2-fold for every 0.12 increase in pCa2+0 (fall in free Ca2+). At pCa2+0 6.1, the activity of the Ca2+ uniport is kinetically limited to 5nmol of Ca2+/min per mg of protein, even when the Ca2+ electrochemical gradient is large. A steady-state cycling of Ca2+ through independent influx and efflux pathways provides a model which is kinetically and thermodynamically consistent with the present observations, and which predicts an extremely precise regulation of pCa2+0 by liver mitochondria in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Wikström M. K., Saris N. E. Effect of inhibitors on the sigmoidicity of the calcium ion transport kinetics in rat liver mitochondria. Biochim Biophys Acta. 1977 Jan 21;464(2):287–294. doi: 10.1016/0005-2736(77)90004-9. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Caldwell P. C. Calcium movements in relation to contraction. Biochem Soc Symp. 1974;(39):29–50. [PubMed] [Google Scholar]
- Azzone G. F., Bragadin M., Pozzan T., Antone P. D. Proton electrochemical potential in steady state rat liver mitochondria. Biochim Biophys Acta. 1977 Jan 6;459(1):96–109. doi: 10.1016/0005-2728(77)90012-3. [DOI] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Massari S., Bragadin M., Dell'Antone P. H+/site ratio and steady state distribution of divalent cations in mitochondria. FEBS Lett. 1977;78(1):21–24. doi: 10.1016/0014-5793(77)80264-0. [DOI] [PubMed] [Google Scholar]
- Bragadin M., Dell'antone P., Pozzan T., Volpato O., Azzone G. F. ESR determination of Mn++ uptake and binding in mitochondria. FEBS Lett. 1975 Dec 15;60(2):354–358. doi: 10.1016/0014-5793(75)80748-4. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Chen C. H., Lehninger A. L. Stoichiometry of H+ ejection during respiration-dependent accumulation of Ca2+ by rat liver mitochondria. J Biol Chem. 1976 Feb 25;251(4):968–974. [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci U S A. 1976 Feb;73(2):437–441. doi: 10.1073/pnas.73.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L., Ramachandran C., Smith R. L. On the mechanism by which inorganic phosphate stimulates mitochondrial calcium transport. FEBS Lett. 1977 Nov 1;83(1):155–158. doi: 10.1016/0014-5793(77)80663-7. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Crompton M., Künzi M., Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem. 1977 Oct 3;79(2):549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- Crompton M., Moser R., Lüdi H., Carafoli E. The interrelations between the transport of sodium and calcium in mitochondria of various mammalian tissues. Eur J Biochem. 1978 Jan 2;82(1):25–31. doi: 10.1111/j.1432-1033.1978.tb11993.x. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., CARAFOLI E., ROSSI C. S., GAMBLE R. L., LEHNINGER A. L. THE STEADY STATE MAINTENANCE OF ACCUMULATED CA++ IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2712–2720. [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Harris E. J., Zaba B. The phosphate requirement for Ca2+-uptake by heart and liver mitochondria. FEBS Lett. 1977 Jul 15;79(2):284–290. doi: 10.1016/0014-5793(77)80804-1. [DOI] [PubMed] [Google Scholar]
- Heaton G. M., Nicholls D. G. The calcium conductance of the inner membrane of rat liver mitochondria and the determination of the calcium electrochemical gradient. Biochem J. 1976 Jun 15;156(3):635–646. doi: 10.1042/bj1560635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson S. M., Pfeiffer D. R., Lardy H. A. Effect of cations and anions on the steady state kinetics of energy-dependent Ca2+ transport in rat liver mitochondria. J Biol Chem. 1976 Sep 10;251(17):5251–5258. [PubMed] [Google Scholar]
- Hutson S. M. Steady state kinetics of energy-dependent Ca2+ uptake in rat liver mitochondria. J Biol Chem. 1977 Jul 10;252(13):4539–4545. [PubMed] [Google Scholar]
- Johnson R. N., Chappell J. B. The transport of inorganic phosphate by the mitochondrial dicarboxylate carrier. Biochem J. 1973 Jul;134(3):769–774. doi: 10.1042/bj1340769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Role of phosphate and other proton-donating anions in respiration-coupled transport of Ca2+ by mitochondria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1520–1524. doi: 10.1073/pnas.71.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Electric charge stoicheiometry of calcium translocation in rat liver mitochondria. FEBS Lett. 1977 Feb 1;73(2):131–136. doi: 10.1016/0014-5793(77)80964-2. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Lanthanide-sensitive calcium-monocarboxylate symport in rat liver mitochondria. FEBS Lett. 1977 Dec 1;84(1):135–140. doi: 10.1016/0014-5793(77)81073-9. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. The lanthanide-sensitive calcium phosphate porter of rat liver mitochondria. FEBS Lett. 1977 May 15;77(2):136–140. doi: 10.1016/0014-5793(77)80220-2. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. [PubMed] [Google Scholar]
- Nicholls D. G. Calcium transport and porton electrochemical potential gradient in mitochondria from guinea-pig cerebral cortex and rat heart. Biochem J. 1978 Mar 15;170(3):511–522. doi: 10.1042/bj1700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Bragadin M., Azzone G. F. Disequilibrium between steady-state Ca2+ accumulation ratio and membrane potential in mitochondria. Pathway and role of Ca2+ efflux. Biochemistry. 1977 Dec 13;16(25):5618–5625. doi: 10.1021/bi00644a036. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Bragadin M., Azzone G. F. The effect of endogenous phosphate on the H+/Mn2+ ratio and the state of Mn2+ in the mitochondrial matrix. Eur J Biochem. 1976 Dec;71(1):93–99. doi: 10.1111/j.1432-1033.1976.tb11093.x. [DOI] [PubMed] [Google Scholar]
- Puskin J. S., Gunter T. E., Gunter K. K., Russell P. R. Evidence for more than one Ca2+ transport mechanism in mitochondria. Biochemistry. 1976 Aug 24;15(17):3834–3842. doi: 10.1021/bi00662a029. [DOI] [PubMed] [Google Scholar]
- Puskin J. S., Gunter T. E. Ion and pH gradients across the transport membrane of mitochondria following Mn ++ uptake in the presence of acetate. Biochem Biophys Res Commun. 1973 Apr 2;51(3):797–803. doi: 10.1016/0006-291x(73)91385-5. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. A kinetic study of mitochondrial calcium transport. Eur J Biochem. 1975 Jul 15;55(3):497–504. doi: 10.1111/j.1432-1033.1975.tb02187.x. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. Electric charge stoichiometry of calcium translocation in mitochondria. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1273–1279. doi: 10.1016/s0006-291x(77)80117-4. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974 Nov 5;13(23):4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Azzone G. F. The mechanism of ion translocation in mitochondria. 4. Coupling of K+ efflux with Ca2+ uptake. Eur J Biochem. 1970 Feb;12(2):328–335. doi: 10.1111/j.1432-1033.1970.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Dunnett S. J. Calcium transport in mitochondria. FEBS Lett. 1970 Sep 18;10(1):1–5. doi: 10.1016/0014-5793(70)80402-1. [DOI] [PubMed] [Google Scholar]
- Sordahl L. A. Effects of magnesium, Ruthenium red and the antibiotic ionophore A-23187 on initial rates of calcium uptake and release by heart mitochondria. Arch Biochem Biophys. 1975 Mar;167(1):104–115. doi: 10.1016/0003-9861(75)90446-4. [DOI] [PubMed] [Google Scholar]
- Stucki J. W., Ineichen E. A. Energy dissipation by calcium recycling and the efficiency of calcium transport in rat-liver mitochondria. Eur J Biochem. 1974 Oct 2;48(2):365–375. doi: 10.1111/j.1432-1033.1974.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta. 1972 Jan 21;256(1):43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]


