Abstract
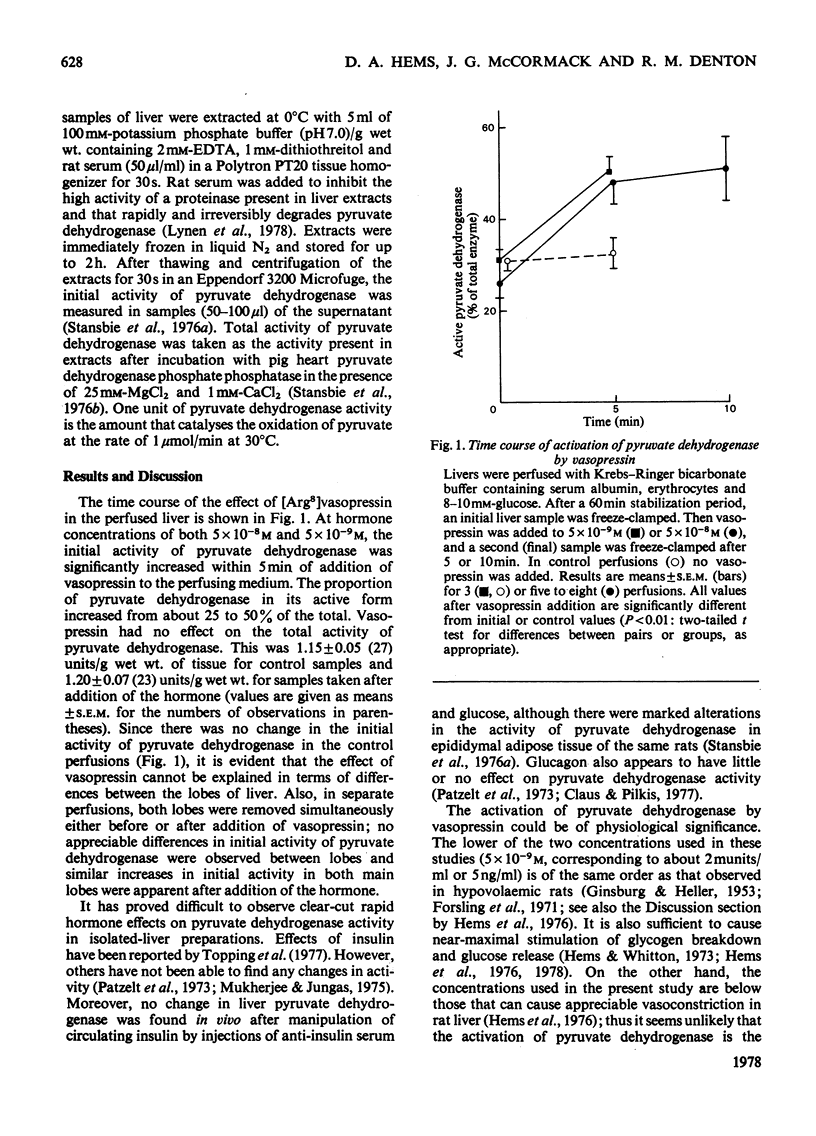
The proportion of pyruvate dehydrogenase in its active form is doubled in rat liver within 5 min of addition of vasopressin to the perfusing medium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Claus T. H., Pilkis S. J. Effect of dichloroacetate and glucagon on the incorporation of labeled substrates into glucose and on pyruvate dehydrogenase in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Jul;182(1):52–63. doi: 10.1016/0003-9861(77)90282-x. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsling M. L., Martin M. J., Burton A. M. The effect of hydration on vasopressin and neurophysin release in the rat. J Endocrinol. 1971 Oct;51(2):413–414. doi: 10.1677/joe.0.0510413. [DOI] [PubMed] [Google Scholar]
- GINSBURG M., HELLER H. Antidiuretic activity in blood obtained from various parts of the cardiovascular system. J Endocrinol. 1953 Jul;9(3):274–282. doi: 10.1677/joe.0.0090274. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Glycogen phosphorylase, glucose output and vasoconstriction in the perfused rat liver. Concentration-dependence of actions of adrenaline, vasopressin and angiotensin II. Biochem J. 1976 Nov 15;160(2):367–374. doi: 10.1042/bj1600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Rodrigues L. M., Whitton P. D. Rapid stimulation by vasopressin, oxytocin and angiotensin II of glycogen degradation in hepatocyte suspensions. Biochem J. 1978 May 15;172(2):311–317. doi: 10.1042/bj1720311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A. Short-term hormonal control of hepatic carbohydrate and lipid catabolism. FEBS Lett. 1977 Aug 15;80(2):237–245. doi: 10.1016/0014-5793(77)80449-3. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Ma G. Y. Metabolic actions of vasopressin, glucagon and adrenalin in the intact rat. Biochim Biophys Acta. 1975 Nov 10;411(1):155–164. doi: 10.1016/0304-4165(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973 Nov;136(3):705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungas R. L. Hormonal regulation of pyruvate dehydrogenase. Metabolism. 1971 Jan;20(1):43–53. doi: 10.1016/0026-0495(71)90058-8. [DOI] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J. Diabetes and the control of pyruvate dehydrogenase in rat heart mitochondria by concentration ratios of adenosine triphosphate/adenosine diphosphate, of reduced/oxidized nicotinamide-adenine dinucleotide and of acetyl-coenzyme A/coenzyme A. Biochem J. 1977 Jun 15;164(3):509–519. doi: 10.1042/bj1640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynen A., Sedlaczek E., Wieland O. H. Partial purification and characterization of a pyruvate dehydrogenase-complex-inactivating enzyme from rat liver. Biochem J. 1978 Feb 1;169(2):321–328. doi: 10.1042/bj1690321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G. Y., Hems D. A. Inhibition of fatty acid synthesis and stimulation of glycogen breakdown by vasopressin in the perfused mouse liver. Biochem J. 1975 Nov;152(2):389–392. doi: 10.1042/bj1520389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Jungas R. L. Activation of pyruvate dehydrogenase in adipose tissue by insulin. Evidence for an effect of insulin on pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1975 May;148(2):229–235. doi: 10.1042/bj1480229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Löffler G., Wieland O. H. Interconversion of pyruvate dehydrogenase in the isolated perfused rat liver. Eur J Biochem. 1973 Feb 15;33(1):117–122. doi: 10.1111/j.1432-1033.1973.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs M., Kirk C. J., Hems D. A. Role of extracellular calcium in the action of vasopressin on hepatic glycogenolysis. FEBS Lett. 1976 Oct 15;69(1):199–202. doi: 10.1016/0014-5793(76)80686-2. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Goheer M. A., Coore H. G., Mayes P. A. Regulation by insulin and free fatty acids of pyruvate dehydrogenase activity in perfused rat liver [proceedings]. Biochem Soc Trans. 1977;5(4):1000–1001. doi: 10.1042/bst0051000. [DOI] [PubMed] [Google Scholar]


