Abstract
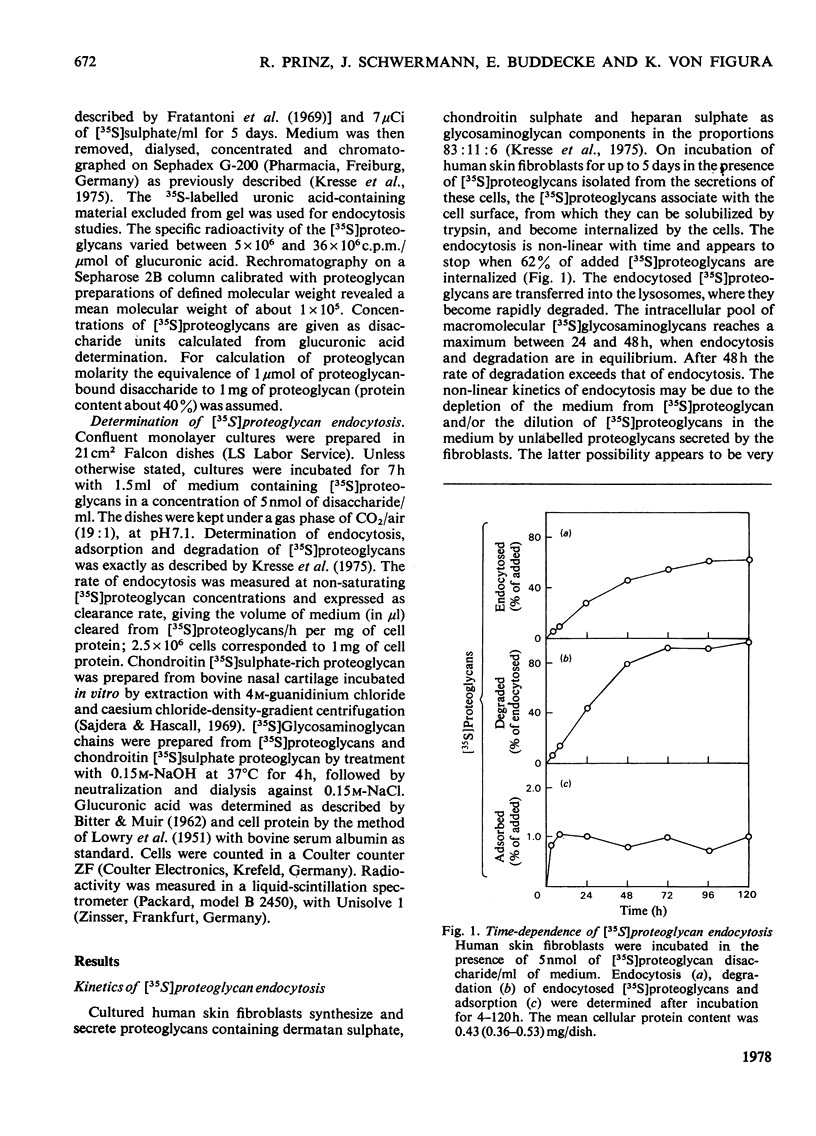
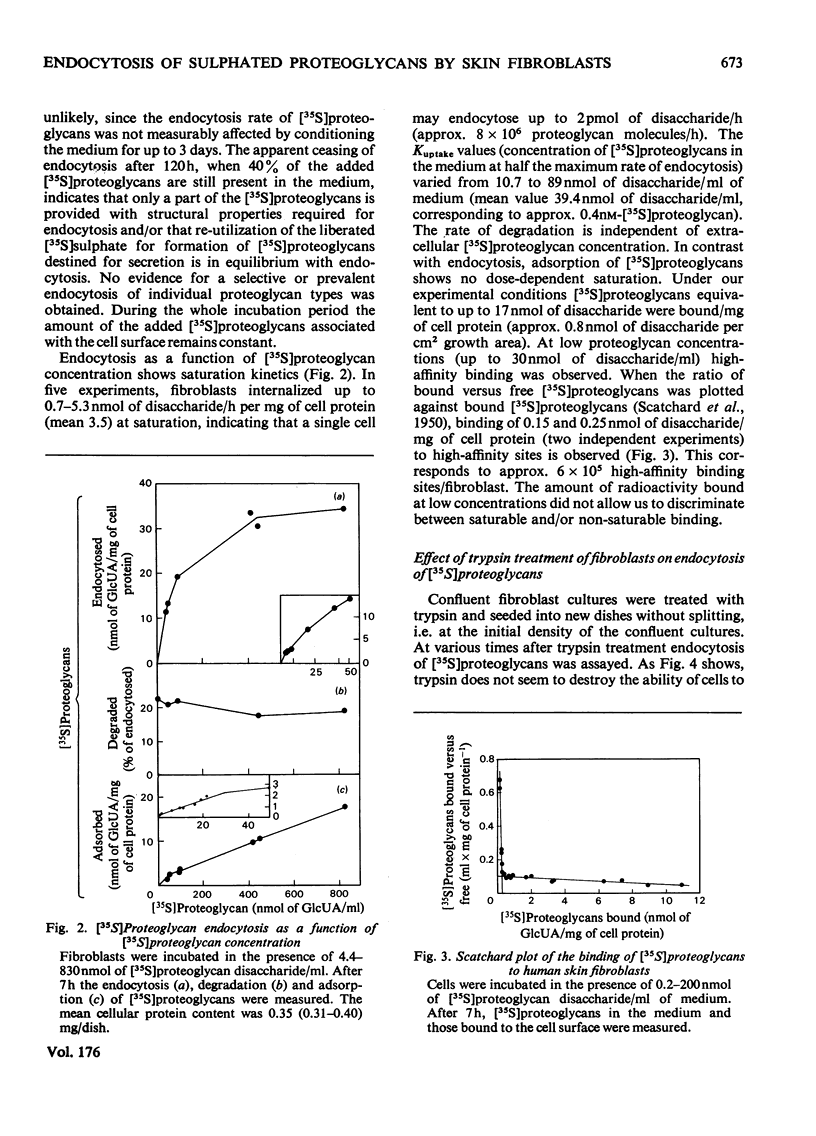
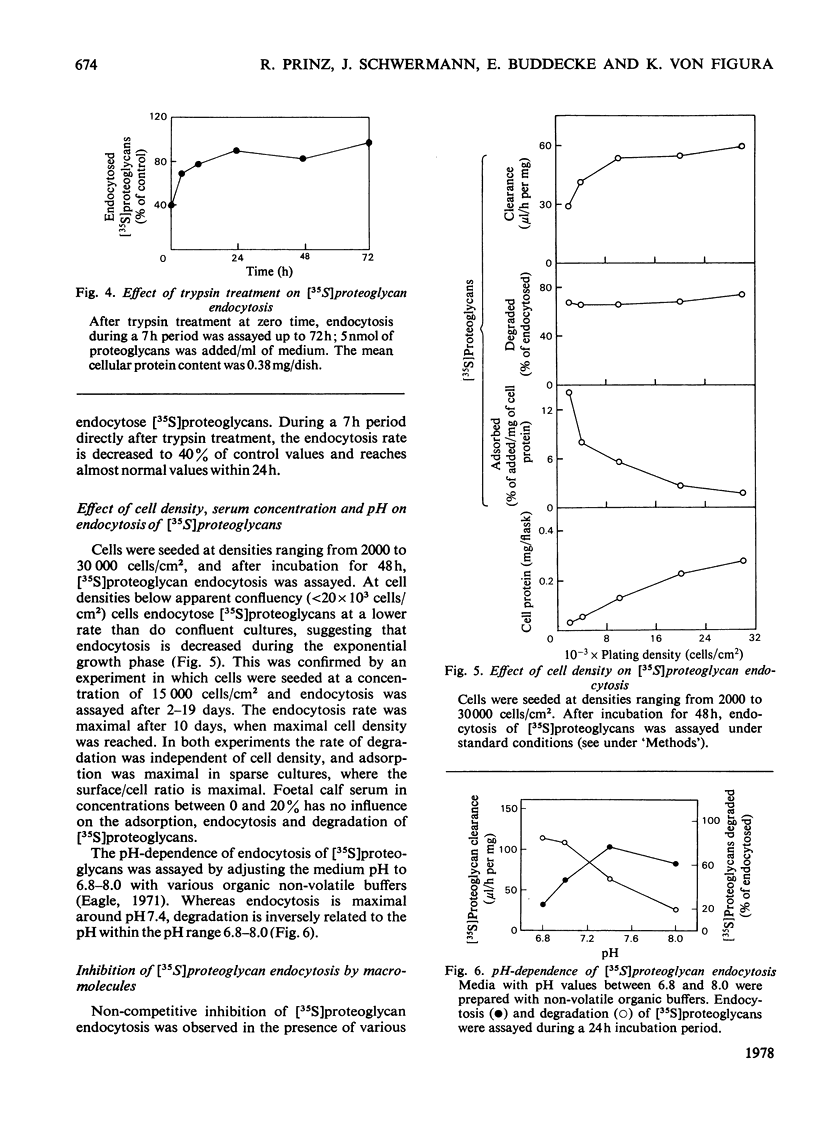
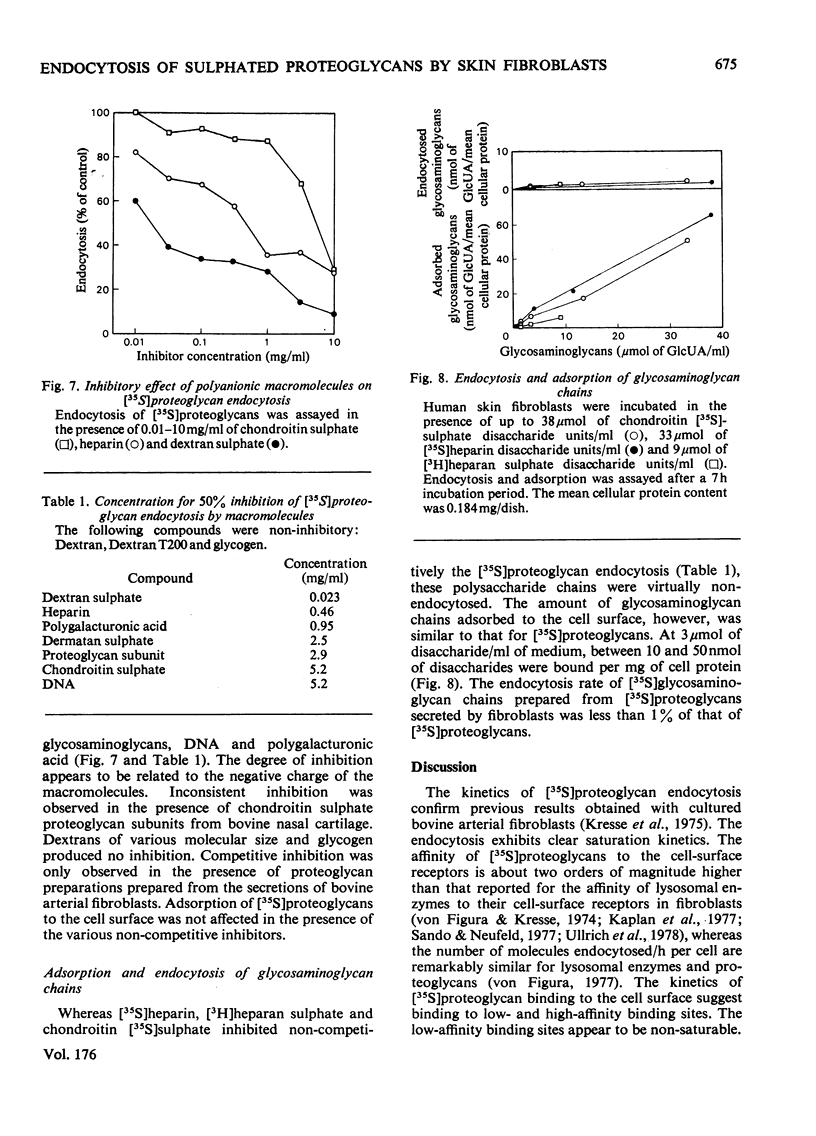
1. Human skin fibroblasts internalize homologous sulphated proteoglycans by adsorptive endocytosis. Endocytosis rate is half maximal when the concentration of the proteoglycans is 0.1 nM. At saturation, a single fibroblast may endocytose up to 8 X 10(6) proteoglycan molecules/h. 2. The kinetics of prote;glycan binding to the cell surface suggest the presence of 6 X 10(5) high-affinity binding sites per cell. The bulk of sulphated proteoglycans associates to low-affinity binding sites on the cell surface. 3. Glycosaminoglycans and other anionic macromolecules inhibit endocytosis of sulphated proteoglycans non-competitively. The lack of interaction of glycosaminoglycans with the cell-surface receptors for sulphated proteoglycans suggests that the protein core of proteoglycans is essential for binding to the cell surface. 4. The effects of trypsin, cell density, serum concentration and medium pH on endocytosis and degradation of endocytosed sulphated proteoglycans is described. 5. A comparison of the number of the high-affinity binding sites and the number of molecules endocytosed with respect to time suggests a recycling of the proteoglycan receptors between the cell surface and the endocytotic vesicles and/or the lysosomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler and Hunter syndromes. II. Deficiency of specific factors involved in mucopolysaccharide degradation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):360–366. doi: 10.1073/pnas.64.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORHAM L. W., WAYMOUTH C. DIFFERENTIATION IN VITRO OF EMBRYONIC CARTILAGE AND BONE IN A CHEMICALLY-DEFINED MEDIUM. Proc Soc Exp Biol Med. 1965 May;119:287–290. doi: 10.3181/00379727-119-30160. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse H., von Figura K., Buddecke E., Fromme H. G. Metabolism of sulfated glycosaminoglycans in cultivated bovine arterial cells. I. Characterization of different pools of sulfated glycosaminoglycans. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):929–941. doi: 10.1515/bchm2.1975.356.s1.929. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lie S. O., McKusick V. A., Neufeld E. F. Simulation of genetic mucopolysaccharidoses in normal human fibroblasts by alteration of pH of the medium. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2361–2363. doi: 10.1073/pnas.69.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., Trouet A. Recycling of fibroblast plasma-membrane antigens internalized during endocytosis [proceedings]. Biochem Soc Trans. 1977;5(4):1164–1167. doi: 10.1042/bst0051164. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Weber E., Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. doi: 10.1042/bj1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K. Human alpha-n-acetylglucosaminidase. 2. Activity towards natural substrates and multiple recognition forms. Eur J Biochem. 1977 Nov 1;80(2):535–542. doi: 10.1111/j.1432-1033.1977.tb11909.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Kresse H. Quantitative aspects of pinocytosis and the intracellular fate of N-acetyl-alpha-D-glucosaminidase in Sanfilippo B fibroblasts. J Clin Invest. 1974 Jan;53(1):85–90. doi: 10.1172/JCI107563. [DOI] [PMC free article] [PubMed] [Google Scholar]


