Abstract
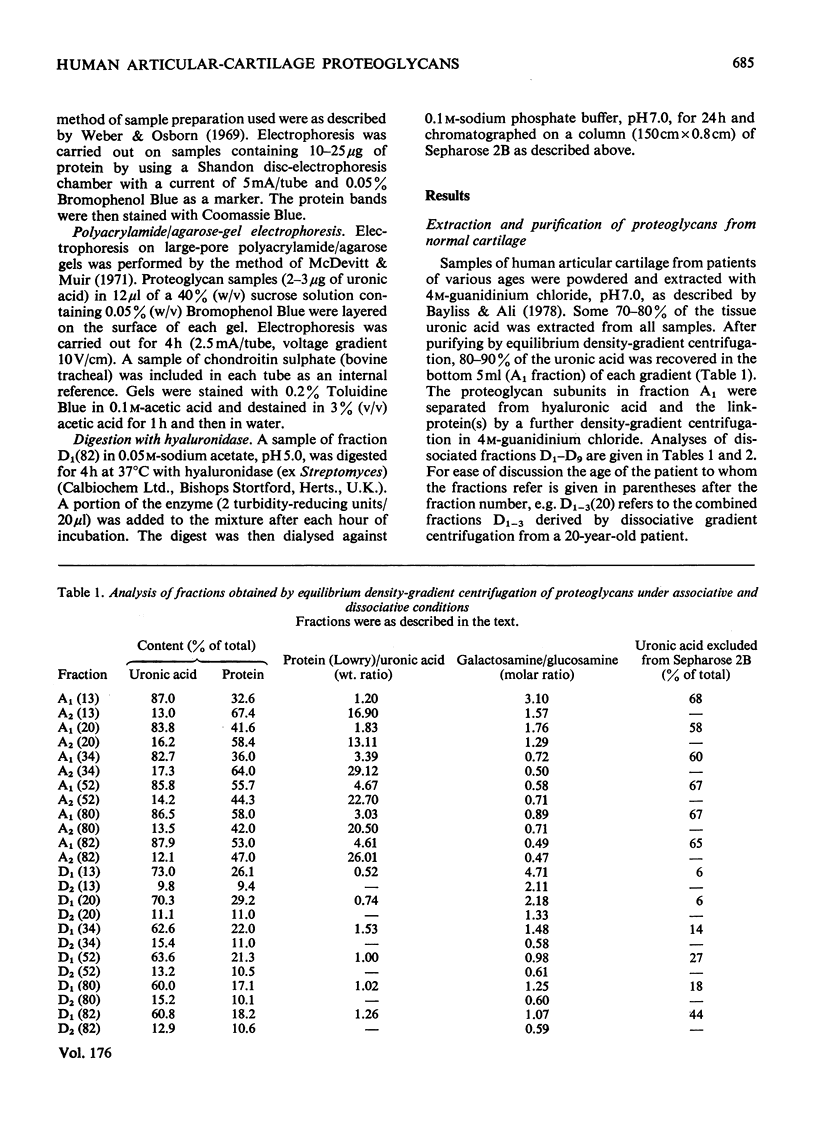
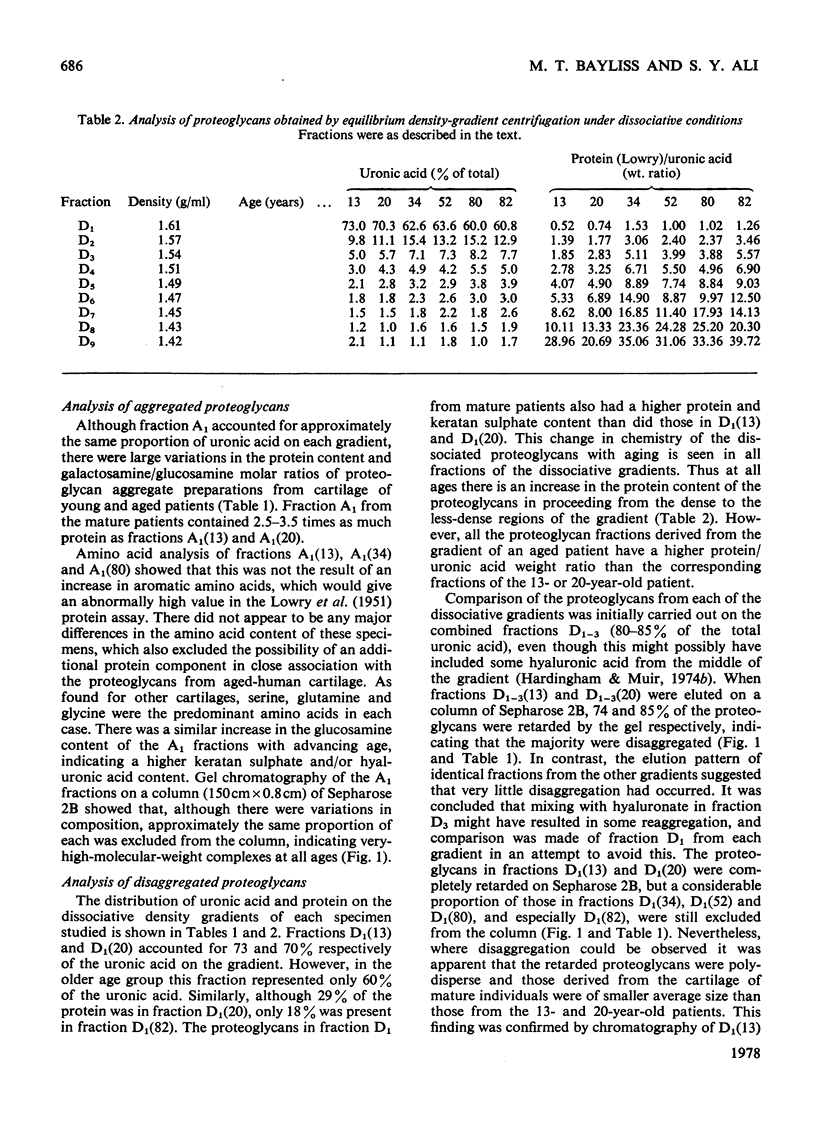
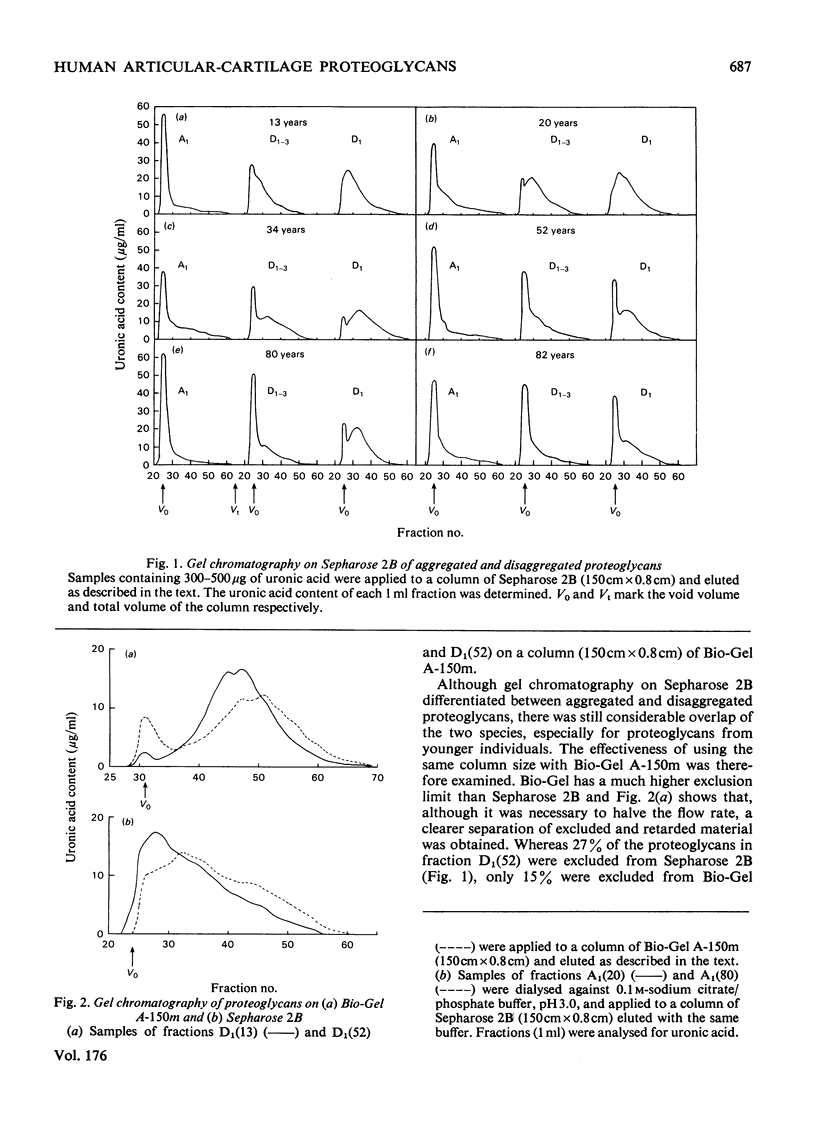
1. Analysis of the purified proteoglycans extracted from normal human articular cartilage with 4M-guanidinium chloride showed that there was an age-related increase in their content of protein and keratan sulphate. 2. The hydrodynamic size of the dissociated proteoglycans also decreased with advancing age, but there was little change in the proportion that could aggregate. 3. Results suggested that some extracts of aged-human cartilage had an increased content of hyaluronic acid compared with specimens from younger patients. 4. Dissociated proteoglycans, from cartilage of all age groups, bind to hyaluronic acid and form aggregates in direct proportion to the hyaluronic acid concentration. 5. Electrophoretic heterogeneity of the dissociated proteoglycans was demonstrated on polyacrylamide/agarose gels. The number of proteoglycan species observed was also dependent on the age of the patient.
Full text
PDF

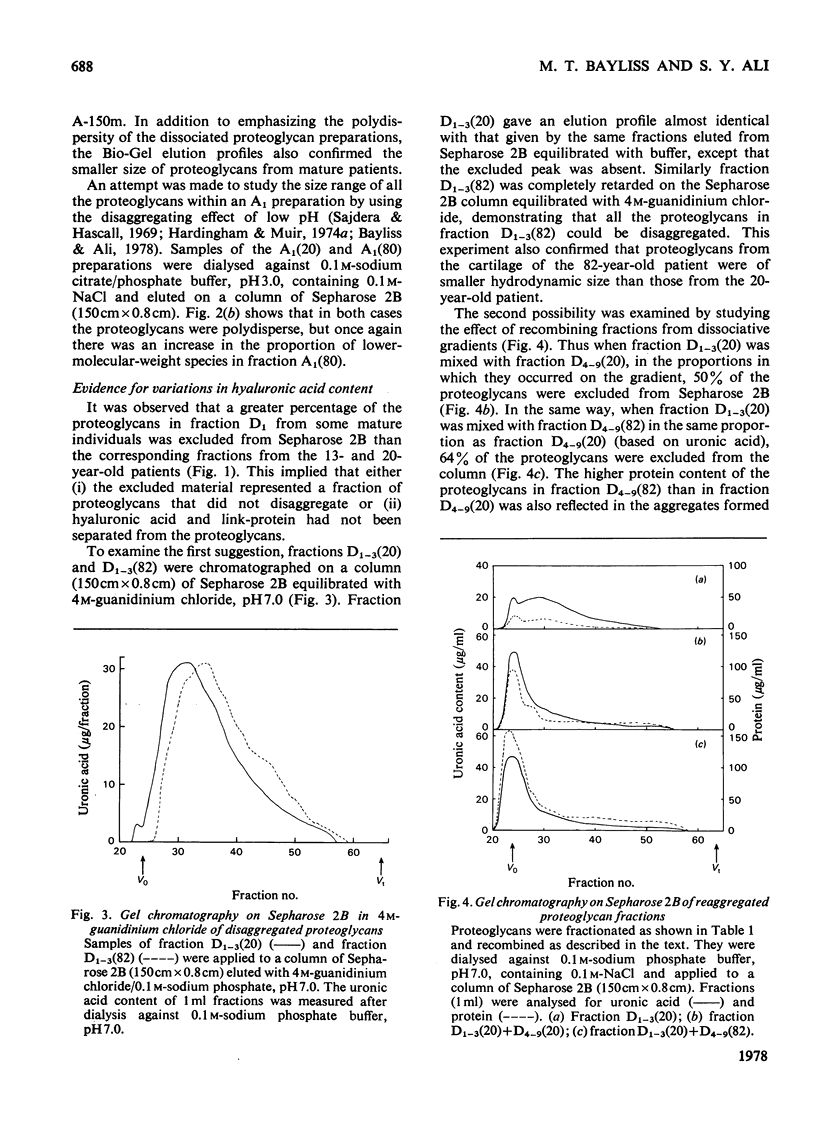

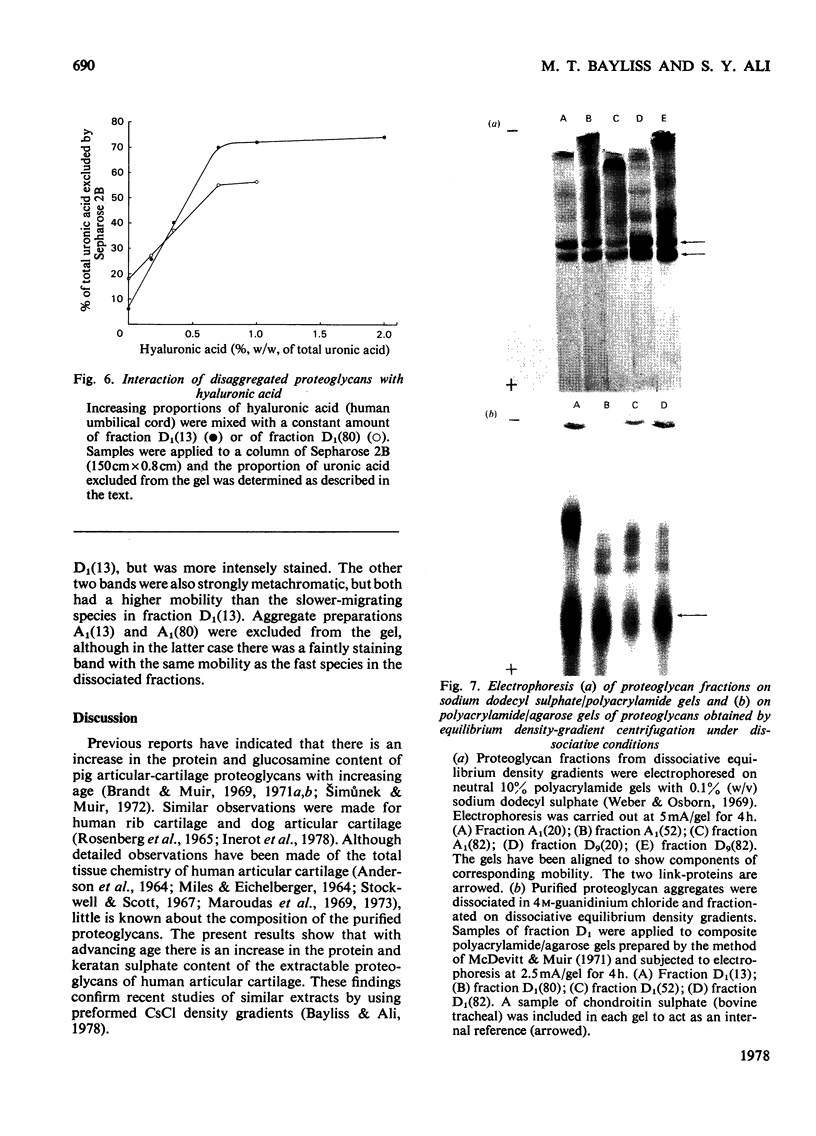



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON C. E., LUDOWIEG J., HARPER H. A., ENGLEMAN E. P. THE COMPOSITION OF THE ORGANIC COMPONENT OF HUMAN ARTICULAR CARTILAGE. RELATIONSHIP TO AGE AND DEGENERATIVE JOINT DISEASE. J Bone Joint Surg Am. 1964 Sep;46:1176–1183. [PubMed] [Google Scholar]
- Armstrong C. G., Bahrani A. S., Gardner D. L. Alteration with age in compliance of human femoral-head cartilage. Lancet. 1977 May 21;1(8021):1103–1104. doi: 10.1016/s0140-6736(77)92356-x. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Characterization of protein-polysaccharides of articular cartilage from mature and immature pigs. Biochem J. 1969 Oct;114(4):871–876. doi: 10.1042/bj1140871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Heterogeneity of protein-polysaccharides of porcine articular cartilage. The chondroitin sulphate proteins associaterd with collagen. Biochem J. 1971 Aug;123(5):747–755. doi: 10.1042/bj1230747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Heterogeneity of protein-polysaccharides of porcine articular cartilage. The sequential extraction of chondroitin sulphate-proteins with iso-osmotic neutral sodium acetate. Biochem J. 1971 Jan;121(2):261–270. doi: 10.1042/bj1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILES J. S., EICHELBERGER L. BIOCHEMICAL STUDIES OF HUMAN CARTILAGE DURING THE AGING PROCESS. J Am Geriatr Soc. 1964 Jan;12:1–20. doi: 10.1111/j.1532-5415.1964.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Maroudas A. I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976 Apr 29;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975 Jun;12(3-4):233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- Maroudas A., Evans H., Almeida L. Cartilage of the hip joint. Topographical variation of glycosaminoglycan content in normal and fibrillated tissue. Ann Rheum Dis. 1973 Jan;32(1):1–9. doi: 10.1136/ard.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Muir H., Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969 May 6;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
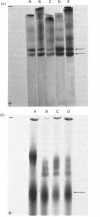
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970 Aug;52(3):554–563. [PubMed] [Google Scholar]
- Ogston A. G., Wells J. D. The osmotic properties of sulphoethyl-sephadex. A model for cartilage. Biochem J. 1972 Jul;128(3):685–690. doi: 10.1042/bj1280685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. Heterogeneity of proteoglycans from adult human costal cartilage [proceedings]. Biochem Soc Trans. 1978;6(1):244–246. doi: 10.1042/bst0060244. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. The stability of bovine nasal cartilage proteoglycans during isolation and storage. Biochim Biophys Acta. 1977 Jun 23;498(1):176–188. doi: 10.1016/0304-4165(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Perricone E., Palmoski M. J., Brandt K. D. Failure of proteoglycans to form aggregates in morphologically normal aged human hip cartilage. Arthritis Rheum. 1977 Sep-Oct;20(7):1372–1380. doi: 10.1002/art.1780200711. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Johnson B., Schubert M. Proteinpolysaccharides from human articular and costal cartilage. J Clin Invest. 1965 Oct;44(10):1647–1656. doi: 10.1172/JCI105271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Wolfenstein-Todel C., Margolis R., Pal S., Strider W. Proteoglycans from bovine proximal humeral articular cartilage. Structural basis for the polydispersity of proteoglycan subunit. J Biol Chem. 1976 Oct 25;251(20):6439–6444. [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., Hatt M., Mason R. M. Physicochemical properties of cartilage proteoglycans extracted by lanthanum chloride. Biochim Biophys Acta. 1978 Apr 3;539(4):445–458. doi: 10.1016/0304-4165(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Roughley P. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan heterogeneity and the pathway of proteolytic degradation. Biochem J. 1977 Dec 1;167(3):639–646. doi: 10.1042/bj1670639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Simůnek Z., Muir H. Changes in the protein-polysaccharides of pig articular cartilage during prenatal life, development and old age. Biochem J. 1972 Feb;126(3):515–523. doi: 10.1042/bj1260515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P. Gel electrophoretic studies on proteoglycans and collagen of abnormal human growth cartilage: proteoglycan abnormalities in pseudoachondroplasia and in Kniest's disease. Pediatr Res. 1975 Oct;9(10):779–782. doi: 10.1203/00006450-197510000-00006. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) articular cartilage. Biochem J. 1977 Apr 1;163(1):103–109. doi: 10.1042/bj1630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Distribution of acid glycosaminoglycans in human articular cartilage. Nature. 1967 Sep 23;215(5108):1376–1378. doi: 10.1038/2151376a0. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Powell S., Broadhurst J., Sordillo E., Sotman S. The formation of a stable complex between dissociated proteoglycan and hyaluronic acid in the absence of a link protein. Biochem J. 1976 Aug 1;157(2):503–506. doi: 10.1042/bj1570503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]