Abstract
Rationale
Despite World Health Organization (WHO) guidelines for preventing, detecting, and treating postpartum hemorrhage (PPH), effective implementation has lagged.
Objectives
To evaluate the clinical benefits and harms of implementation strategies used to promote adherence to WHO clinical guidelines for the prevention, detection, and treatment of PPH.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, and two trial registries, along with reference checking, citation searching, and contact with study authors. The latest search date was 25 April 2024.
Eligibility criteria
We included randomized controlled trials (RCTs), including cluster, pragmatic, and stepped‐wedge designs, and non‐randomized studies of interventions (NRSIs), including interrupted time series (ITS) studies, controlled before‐after (CBA) studies, and follow‐up (cohort) studies containing concurrent controls that focused on or described implementation strategies of WHO guidelines for the prevention, detection, and treatment of PPH. Participants were birth attendants and people giving birth in a hospital or healthcare facility. We excluded studies that did not implement a WHO PPH recommendation, had no comparator group, or did not report clinical/implementation outcomes.
Outcomes
Our critical outcomes were: adherence to WHO‐recommended guidelines for PPH prevention, detection, and treatment; PPH ≥ 500 mL; PPH ≥ 1000 mL; additional uterotonics within 24 hours after birth; blood transfusions; maternal death; severe morbidities (major surgery; admission to intensive care unit [ICU]); and adverse effects (variable and related to the clinical intervention) during hospitalization for birth.
Our important outcomes were: breastfeeding at discharge; implementation outcomes such as acceptability, adoption, appropriateness, feasibility, fidelity, implementation cost, penetration, and sustainability of the implementation strategy; and health professional outcomes such as knowledge and skill.
Risk of bias
We used the RoB 2 and ROBINS‐I tools to assess risk of bias in RCTs and NRSIs, respectively.
Synthesis methods
Two review authors independently selected studies, performed data extraction, and assessed risk of bias and trustworthiness. Due to the nature of the data, we reported relevant results for each comparison and outcome but did not attempt quantitative synthesis. We used GRADE to assess the certainty of evidence.
Included studies
We included 13 studies (9 cluster‐RCTs and 4 NRSIs) with a total of 1,027,273 births and more than 4373 birth attendants. The included studies were conducted in 17 different countries. Most trials were conducted in resource‐limited settings. None of the included studies reported data on the use of additional uterotonics within 24 hours after birth or adverse effects.
Synthesis of results
Single‐component implementation strategies versus usual care for PPH prevention, detection, and treatment
We do not know if single‐component implementation strategies have any effect on adherence to WHO PPH prevention recommendations, PPH ≥ 500 mL, PPH ≥ 1000 mL, or blood transfusion (very low‐certainty evidence).
Low‐certainty evidence suggests that single‐component implementation strategies may have little to no effect on maternal death (86,788 births, 3 trials); may increase severe morbidity related to ICU admission (26,985 births, 1 trial); and may reduce severe morbidity related to surgical outcomes (26,985 births, 1 trial).
No trials in this comparison measured the effect on adherence to WHO treatment guidelines.
Multicomponent implementation strategies versus usual care for PPH prevention, detection, and treatment
We do not know if multicomponent implementation strategies have any effect on adherence to WHO PPH treatment recommendations, PPH ≥ 500 mL, blood transfusion, or severe morbidity relating to surgical outcomes (very low‐certainty evidence).
Multicomponent implementation strategies may have little to no effect on maternal death (274,008 births, 2 trials; low‐certainty evidence) compared to usual care.
No trials in this comparison measured the effect on adherence to WHO PPH prevention recommendations, PPH ≥ 1000 mL, or severe morbidity (outcomes related to ICU admission).
Multicomponent implementation strategies versus enhanced usual care for PPH prevention, detection, and treatment
Low‐certainty evidence suggests that multicomponent implementation strategies may improve adherence to WHO PPH prevention recommendations (14,718 births, 2 trials) and adherence to WHO PPH treatment recommendations (356,913 births, 2 trials) compared to enhanced usual care.
Multicomponent implementation strategies probably have little to no effect on maternal death (224,850 births, 2 trials; moderate‐certainty evidence), severe morbidity related to ICU admission (224,850 births, 2 trials; moderate‐certainty evidence), and surgical morbidity (210,132 births, 1 trial; moderate‐certainty evidence) compared to enhanced usual care.
We do not know if multicomponent implementation strategies affect PPH ≥ 500 mL, PPH ≥ 1000 mL, or blood transfusion (very low‐certainty evidence).
Authors' conclusions
Multicomponent implementation strategies may improve adherence to WHO PPH prevention and treatment recommendations, but they probably result in little to no difference in ICU admissions, surgical morbidity, or maternal death. The majority of available evidence is of low to very low certainty, thus we cannot draw any robust conclusions on the effects of implementation strategies for WHO guidelines to prevent, detect, and treat PPH. While all included studies used the implementation strategy of 'train and educate,' the effects seem to be limited when used as a single strategy. Additional research using pragmatic, hybrid effectiveness‐implementation study designs that measure implementation outcomes simultaneously alongside clinical outcomes would be beneficial to understand contextual factors, barriers, and facilitators that affect implementation.
Funding
This Cochrane review had no dedicated external funding. Dr Rose Molina, who is employed by Beth Israel Deaconess Medical Center, received funding from Ariadne Labs (Harvard T.H. Chan School of Public Health, Brigham and Women's Hospital) for her time. As a funder, Ariadne Labs had no involvement in the development of the protocol or conduct of the review. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of Ariadne Labs.
Registration
Registration: PROSPERO (CRD42024563802) available via https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024563802
Keywords: Female, Humans, Pregnancy, Bias, Blood Transfusion, Blood Transfusion/standards, Blood Transfusion/statistics & numerical data, Controlled Before-After Studies, Guideline Adherence, Interrupted Time Series Analysis, Maternal Mortality, Oxytocics, Oxytocics/therapeutic use, Postpartum Hemorrhage, Postpartum Hemorrhage/diagnosis, Postpartum Hemorrhage/mortality, Postpartum Hemorrhage/prevention & control, Postpartum Hemorrhage/therapy, Practice Guidelines as Topic, Randomized Controlled Trials as Topic, World Health Organization
Plain language summary
What are the best strategies to implement World Health Organization (WHO) recommendations to prevent, detect, and treat postpartum hemorrhage?
Key messages
Multicomponent implementation strategies may improve adherence to World Health Organization (WHO) postpartum hemorrhage (PPH) prevention recommendations and probably do not make a difference to intensive care unit (ICU) admissions, need for additional surgeries, or death of the mother. We do not know if multicomponent implementation strategies affect blood loss or blood transfusion.
We do not know if single‐component implementation strategies affect adherence to WHO PPH prevention recommendations, blood loss, or blood transfusion. Single‐component implementation strategies may not make a difference to the death of the mother, may increase ICU admissions, and may reduce the need for additional surgeries.
The small number of studies and differences in data collected across included studies limited our ability to draw any conclusions on effective implementation strategies; however, there were varying degrees of success with identical implementation strategies in different studies, highlighting the need for future research in this area.
What is postpartum hemorrhage (PPH)?
Postpartum hemorrhage is typically defined as blood loss greater than 500 mL within 24 hours after birth.
How is PPH prevented, diagnosed, and treated?
WHO guidelines recommend oxytocin administration immediately after birth to prevent PPH. Some birth facilities use blood collection drapes and scales to measure blood loss; however, many do not have access to these supplies. The treatment of PPH varies based on the severity, underlying cause, and available resources. Most cases of PPH are treated with medications that cause the uterus to contract. In women who do not respond to this medication, uterine balloon tamponade (where a balloon is inflated in the uterus to compress blood vessels and stop bleeding) or surgery is needed.
What are implementation strategies?
Implementation strategies are specific techniques used to increase the acceptance, uptake, and sustainability of a clinical practice or program. Examples include engaging individuals and leaders, changing infrastructure, and training and educating and/or supporting birth attendants.
How are implementation strategies used to prevent, diagnose, and treat PPH?
A wide range of implementation strategies have been used in clinical practice to prevent, diagnose, and treat PPH. Strategies include training and educating skilled birth attendants in evidence‐based practices, introducing new equipment to birth facilities, and developing reporting systems to audit health records and provide feedback to healthcare workers.
What did we want to find out?
We wanted to know which, if any, implementation strategies of WHO PPH recommendations are effective in facility‐based childbirth settings.
What did we do?
We searched for studies that looked at the effects of implementation strategies of WHO PPH recommendations by birth attendants on people who gave birth in a health facility. We summarized the results of the studies and rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find out?
We included 13 studies, which were categorized into three groups based on the implementation strategies used: (1) single strategy versus usual care, (2) multiple strategies versus usual care, and (3) multiple strategies versus enhanced usual care.
We do not know if single‐component implementation strategies affect adherence to WHO PPH prevention recommendations, blood loss, or blood transfusion. Single‐component implementation strategies may not make a difference to maternal death, may reduce the need for additional surgeries, but may also increase ICU admissions.
Multicomponent implementation strategies may improve adherence to PPH prevention recommendations and probably do not make a difference to ICU admissions, need for additional surgeries, or maternal death. We do not know if multicomponent implementation strategies affect blood loss or blood transfusion.
We found that the same implementation strategies and study approach can increase adherence to WHO guidelines in one setting, not make any difference in another, and even reduce adherence in others.
Many studies lacked a comprehensive framework that linked implementation efforts with adherence to WHO recommendations and patient outcomes; it is doubtful that multicomponent intervention could address all factors that contribute to PPH‐related illness or death. It remains unknown whether multiple strategies work in a real‐world setting.
What are the limitations of the evidence?
The level of detail of implementation strategies varied, preventing the combining of studies. There were also differences in the people included in the studies according to facility level, volume of births, and type of delivery. Different study contexts made it difficult to measure the true impact of implementation strategies on outcomes.
How up‐to‐date is this evidence?
The evidence is current to 25 April 2024.
Summary of findings
Summary of findings 1. Single‐component implementation strategies versus usual care for PPH prevention, detection, and treatment.
|
Patients or population: pregnant people admitted to healthcare facilities for birth and birth attendants Settings: hospital or healthcare facility Intervention: train and educate implementation strategy Comparison: usual care | ||||||
| Outcome | No. of studies | Description of results | Implementation strategy domain | No. of participants or births (studies, clusters) | Certainty of the evidence (GRADE) | Summary statement |
| Adherence to WHO PPH prevention recommendation during study period1 | 1 | Klokkenga 2019 reported an increase to 1 recommendation (P3, Table 2) lowering the episiotomy rate (to 1% in implementation group vs 10% control), and no increase to another (P2, Table 2), with no difference in the use of uterotonics in the implementation arm vs control (97% vs 96%, respectively). | Train and educate | 3411 births (1 study, 15 clusters) | VERY LOWa,b,c | We do not know if single‐component implementation strategies have any effect on the adherence to WHO PPH prevention recommendations. |
| Adherence to WHO PPH treatment recommendation | No studies reported this outcome. | |||||
| PPH ≥ 500 mL within 24 hours after birth | 2 | Klokkenga 2019 reported a reduction in PPH (OR 0.86, 95% CI 0.59 to 1.25). Naidoo 2017 reported a reduction in PPH (IRR 0.91, 95% CI 0.66 to 1.28). |
Train and educate | 30,396 births (2 studies, 62 clusters) | VERY LOWc,d | We do not know if single‐component implementation strategies have any effect on PPH ≥ 500 mL. |
| PPH ≥ 1000 mL within 24 hours after birth | 1 | Klokkenga 2019 reported a reduction in PPH (OR 0.95, 95% CI 0.65 to 1.40). | Train and educate | 3411 births (1 study, 15 clusters) | VERY LOWc,d,e | We do not know if single‐component implementation strategies have any effect on PPH ≥ 1000 mL. |
| Additional uterotonics within 24 hours after birth | No studies reported this outcome. | |||||
| Blood transfusion during hospitalization for birth | 1 | Klokkenga 2019 reported no difference in the implementation arm (14/1665) vs control (27/1746). | Train and educate | 3411 births (1 study, 15 clusters) | VERY LOWb,c | We do not know if single‐component implementation strategies have any effect on blood transfusion. |
| Maternal death until discharge for birth hospitalization and up to 42 days after birth | 3 | 3 RCTs reported no difference between implementation [I] and control [C] arms. Klokkenga 2019: [I] 3 deaths/1665 women vs [C] 3 deaths/1746 women Naidoo 2017:
van de Ven 2017:
|
Train and educate | 86,788 births (3 studies, 86 clusters) | LOWc,f | Single‐component implementation strategies may have little or no effect on maternal death. |
| Severe morbidity (outcomes related to ICU admission) during birth hospitalization | 1 | Naidoo 2017 reported increased referral to higher level of care:
|
Train and educate | 26,985 births (1 study, 47 clusters) | LOWe,g | Single‐component implementation strategies may increase severe morbidity (outcomes related to ICU admission). |
| Severe morbidity (surgical outcomes) during birth hospitalization | 1 | Naidoo 2017 found a decrease in unscheduled returns to the operating theater:2
|
Train and educate | 26,985 births (1 study, 47 clusters) | LOWe, g | Single‐component implementation strategies may reduce severe morbidity (surgical outcomes). |
| Adverse effects (variable and related to the intervention) during hospitalization for birth | No studies reported this outcome. | |||||
| CI: confidence interval; ICU: intensive care unit; IRR: incidence rate ratio; OR: odds ratio; PPH: postpartum hemorrhage; RCT: randomized controlled trial; WHO: World Health Organization. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious imprecision, as there were no measures of variation and few participants. bDowngraded one level due to serious indirectness; single RCT with only 70 participants. A single provider type contributed to the outcome. cDowngraded two levels due to high risk of bias. dDowngraded two levels due to very serious imprecision; wide CIs, both the lower and upper bound cross thresholds for clinically important effects. eDowngraded one level due to indirectness; one study reporting on a very rare outcome, and results may not be generalizable. fNot downgraded for imprecision. One study reports a wide CI; however, this outcome is very rare. gDowngraded one level due to some concerns related to risk of bias.
1Klokkenga 2019 measured adherence nine weeks' post‐implementation of the intervention. 2van de Ven 2017 measured a similar outcome to morbidity (surgical outcomes) and found an increase in the first quarter in this composite outcome and no change thereafter. This has not been synthesized with the other RCTs, as it was presented as a composite outcome in the trial report, and the outcome definition differs significantly from others. Additionally, we could not conduct a risk of bias assessment on this result as it is a composite outcome made up of multiple outcome measurements.
1. WHO guideline recommendations for the prevention, diagnosis, and treatment of PPH included, by study*.
| WHO recommendations for the prevention of PPH | ||||||||||||||
| Study ID | P1. Third stage uterotonic administration1 | P2. Prophylactic oxytocin2 | P3. Episiotomy3 | P4. Second stage techniques4 | P5. Avoid sustained uterine massage5 | P6. Late cord clamping**6 | P7. CCT during prolonged third stage7 | P8. CCT with a skilled birth attendant8 | P9. CCT without a skilled birth attendant**9 | P10. CCT during CS10 | ||||
| Randomized studies | ||||||||||||||
| Althabe 2008 | Included | Included | Included | Included | ||||||||||
| Al‐beity 2019 | Included | Included | Included | Included | ||||||||||
| Deneux‐Tharaux 2010 | Included | |||||||||||||
| Evans 2018 | Included | Included | Included | Included | Included | Included | ||||||||
| Gallos 2024 | Included | Included | ||||||||||||
| Hanson 2021 | Included | Included | Included | Included | ||||||||||
| Klokkenga 2019 | Included | Included | Included | |||||||||||
| Naidoo 2017 | ||||||||||||||
| van de Ven 2017 | ||||||||||||||
| Non‐randomized studies | ||||||||||||||
| Main 2017 | Included | Included | ||||||||||||
| Mogilevkina 2022 | Included | Included | Included | Included | Included | Included | Included | Included | ||||||
| Liabsuetrakul 2017 | Included | |||||||||||||
| Sloan 2005 | Included | Included | ||||||||||||
| Studies awaiting classification | ||||||||||||||
| Jayanna 2016 | ||||||||||||||
| WHO recommendations for the diagnosis of PPH | ||||||||||||||
| Study ID | D1. Measure of blood loss11 | |||||||||||||
| Randomized studies | ||||||||||||||
| Althabe 2008 | Included | |||||||||||||
| Al‐beity 2019 | Included | |||||||||||||
| Deneux‐Tharaux 2010 | Included | |||||||||||||
| Evans 2018 | Included | |||||||||||||
| Gallos 2024 | Included | |||||||||||||
| Hanson 2021 | Included | |||||||||||||
| Klokkenga 2019 | Included | |||||||||||||
| Naidoo 2017 | Included | |||||||||||||
| van de Ven 2017 | ||||||||||||||
| Non‐randomized studies | ||||||||||||||
| Main 2017 | Included | |||||||||||||
| Mogilevkina 2022 | Included | |||||||||||||
| Liabsuetrakul 2017 | ||||||||||||||
| Sloan 2005 | ||||||||||||||
| Studies awaiting classification | ||||||||||||||
| Jayanna 2016 | ||||||||||||||
| WHO recommendations for the treatment of PPH | ||||||||||||||
| Study ID | T1. PPH treatment bundle12 | T2. TXA13 | T3. External aortic compression14 | T4. IV oxytocin15 | T5. IV ergometrine/prostaglandin16 | T6. Surgical intervention17 | T7. IV fluid resuscitation18 | T8. Uterine massage19 | T9. Bimanual uterine compression20 | T10. UAE21 | T11. Uterine balloon tamponade22 | T12. Avoid uterine packing**23 | T13. Non‐pneumatic anti‐shock garments**24 | T14. Antibiotics for manual removal of placenta**25 |
| Randomized studies | ||||||||||||||
| Althabe 2008 | Included | |||||||||||||
| Al‐beity 2019 | Included | Included | Included | |||||||||||
| Deneux‐Tharaux 2010 | Included | Included | Included | |||||||||||
| Evans 2018 | Included | Included | Included | Included | ||||||||||
| Gallos 2024 | Included | Included | Included | Included | Included | |||||||||
| Hanson 2021 | Included | Included | Included | |||||||||||
| Klokkenga 2019 | Included | Included | Included | |||||||||||
| Naidoo 2017 | Included | |||||||||||||
| van de Ven 2017 | Included | Included | ||||||||||||
| Non‐randomized studies | ||||||||||||||
| Main 2017 | Included | Included | Included | Included | Included | Included | Included | |||||||
| Mogilevkina 2022 | Included | Included | Included | Included | Included | Included | ||||||||
| Liabsuetrakul 2017 | Included | Included | ||||||||||||
| Sloan 2005 | Included | Included | Included | Included | ||||||||||
| Studies awaiting classification | ||||||||||||||
| Jayanna 2016 | ||||||||||||||
| CCT: controlled cord traction is the recommended method for removal of the placenta in cesarean section (WHO recommendations for the prevention and treatment of postpartum hemorrhage, 2012); CS: cesarean section; IV: intravenous; PPH: postpartum hemorrhage; TXA: tranexamic acid; UAE: uterine artery embolism; WHO: World Health Organization | ||||||||||||||
*Bold text denotes WHO recommendation that was directly measured in the study and abstracted in the review. **WHO recommendation was not included in any included study.
1For women in the third stage of labor, uterotonic use improves maternal outcome [67]. 2The use of oxytocin is recommended for the prevention of PPH for all births. In situations where women giving birth vaginally already have IV access, the slow IV administration of 10 international units of oxytocin is recommended in preference to intramuscular administration [67]. 3Routine or liberal use of episiotomy is not recommended for women undergoing spontaneous vaginal birth [68]. 4In the second stage of labor, the use of techniques to reduce perineal trauma and facilitate spontaneous birth improves maternal outcomes [68]. 5Sustained uterine massage is not recommended as an intervention to prevent PPH in women who have received prophylactic oxytocin [69]. 6Late cord clamping (performed after 1 to 3 minutes after birth) is recommended for all births while initiating simultaneous essential newborn care (early cord clamping is not recommended, unless the neonatal is asphyxiated and needs to move immediately for resuscitation) [69]. 7For women with a duration of third stage of labor longer than 30 minutes, the use of cord traction in addition to usual care can improve maternal outcomes [69]. 8In settings where skilled birth attendants are available, CCT is recommended for vaginal births if the care provider and the parturient woman regard a small reduction in blood loss and a small reduction in the duration of third stage of labor as important [69]. 9In settings where skilled birth attendants are unavailable, CCT is not recommended [69]. 10Controlled cord traction is the recommended method for removal of the placenta in cesarean section [69]. 11For all women giving birth, routine objective measurement of postpartum blood loss is recommended to improve the detection and prompt treatment of PPH. Methods to objectively quantify blood loss, such as calibrated drapes for women having vaginal birth, can achieve this [70]. 12A standardized and timely approach to the management of PPH, comprising an objective assessment of blood loss and use of a treatment bundle supported by an implementation strategy, is recommended for all women having a vaginal birth. The care bundle for firstline treatment of PPH should include rapid institution of uterine massage, administration of an oxytocic agent and TXA, IV fluids, examination of the genital tract, and escalation of care [70]. 13Early use of IV TXA (within 3 hours of birth) in addition to standard care is recommended for women with clinically diagnosed PPH following vaginal birth or cesarean section [71]. 14The use of external aortic compression for the treatment of PPH due to uterine atony after vaginal birth is recommended as a temporary measure until appropriate care is available [69]. 15Intravenous oxytocin alone is the recommended uterotonic drug for the treatment of PPH [69]. 16If IV oxytocin is unavailable, or bleeding does not respond to oxytocin, the use of IV ergometrine, oxytocin‐ergometrine fixed dose, or a prostaglandin drug (including sublingual misoprostol, 800 µg) is recommended [69]. 17If other measures have failed and the necessary resources are available, use of uterine artery embolization is recommended as a treatment for PPH due to uterine atony [69]. 18The use of isotonic crystalloids is recommended in preference to the use of colloids for the initial IV fluid resuscitation of women with PPH [69]. 19Uterine massage is recommended for the treatment of PPH [69]. 20The use of bimanual uterine compression is recommended as a temporary measure until appropriate care is available for the treatment of PPH due to uterine atony after vaginal delivery [69]. 21If other measures have failed and the necessary resources are available, use of uterine artery embolization is recommended as a treatment for PPH due to uterine atony [69]. 22If bleeding does not stop despite treatment using uterotonics and other available conservative interventions (e.g. uterine massage, balloon tamponade), use of surgical interventions is recommended [69]. 23The use of uterine packing is not recommended for the treatment of PPH due to uterine atony after vaginal birth [69]. 24The use of non‐pneumatic anti‐shock garments is recommended as a temporary measure until appropriate care is available [69]. 25A single dose of antibiotics (ampicillin or first‐generation cephalosporin) is recommended if manual removal of the placenta is practiced [69].
Summary of findings 2. Multicomponent implementation strategies versus usual care for PPH prevention, detection, and treatment.
|
Patients or population: pregnant people admitted to healthcare facility for birth and birth attendants Settings: hospital or healthcare facility Intervention: multicomponent implementation strategies Comparison: usual care | ||||||
| Outcome | No. of studies | Description of results | Summary of implementation strategy domains used | No. of participants or births (studies, clusters) | Certainty of the evidence (GRADE) | Summary statement |
| Adherence to WHO PPH prevention recommendation | No studies reported this outcome. | |||||
| Adherence to WHO PPH treatment recommendation during study period1 | 2 | 2 RCTs (Al‐beity 2019; Hanson 2021) reported adherence to 1 recommendation (T4, Table 2). Al‐beity 2019 reported an increase in oxytocin treatment (baseline 92.4%; DiD in slopes 5.20, 95% CI 1.40 to 8.90), whereas Hanson 2021 reported a decrease (baseline 79.0%; DiD in slopes −4.02, 95% CI −6.74 to −1.30). |
|
274,008 births (2 studies, 38 clusters) | VERY LOWa,b | We do not know if multicomponent implementation strategies have any effect on adherence to WHO PPH treatment recommendation. |
| PPH ≥ 500 mL within 24 hours after birth | 2 | Al‐beity 2019 reported a decrease in PPH (DiD in slopes −0.30, 95% CI −0.70 to 0.10). Hanson 2021 reported an increase in PPH (DiD in slopes 0.53, 95% CI 0.15 to 0.92). |
|
274,008 births (2 studies, 38 clusters) | VERY LOWa,b,2 | We do not know if multicomponent implementation strategies have any effect on PPH ≥ 500 mL. |
| PPH ≥ 1000 mL within 24 hours after birth | No studies reported this outcome. | |||||
| Additional uterotonics within 24 hours after birth | No studies reported this outcome. | |||||
| Blood transfusion during hospitalization for birth | 2 | Al‐beity 2019 reported a decrease in blood transfusion (DiD in slopes −8.0, 95% CI −12.6 to −3.4). Hanson 2021 reported no difference (DiD in slopes −0.35, 95% CI −2.59 to 1.88). |
|
274,008 births (2 studies, 38 clusters) | VERY LOWa,b,3 | We do not know if multicomponent implementation strategies have any effect on blood transfusion. |
| Maternal death until discharge for birth hospitalization and up to 42 days after birth | 2 | 2 RCTs reported no difference in maternal death between implementation [I] and control [C] arms. Al‐beity 2019:
Hanson 2021:
|
|
274,008 births (2 studies, 38 clusters) | LOWb,c,4 | Multicomponent implementation strategies may have little or no effect on maternal death. |
| Severe morbidity (outcomes related to ICU admission) during hospitalization for birth | No studies reported this outcome. | |||||
| Severe morbidity (surgical outcomes) during hospitalization for birth | 2 | 2 RCTs reported a reduction in PPH near‐misses among women who suffered PPH during health facility delivery. Hanson 2021:
Al‐beity 2019:
|
|
274,008 births (2 studies, 38 clusters) | VERY LOWa,b,c | We do not know if multicomponent implementation strategies have any effect on severe morbidity (surgical outcomes). |
| Adverse effects (variable and related to the intervention) during hospitalization for birth | No studies reported this outcome. | |||||
| CI: confidence interval; DiD: difference in differences; ICU: intensive care unit; ITS: interrupted time series assessing long‐term effect difference between implementation arm and comparison district (ITS analysis); NRSI: non‐randomized study of interventions; PPH: postpartum hemorrhage; RCT: randomized controlled trial; WHO: World Health Organization. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for inconsistency, as the two trials showed different effects. bDowngraded two levels due to high risk of bias. cNot downgraded for imprecision. The outcome is very rare and, when considered in absolute terms, represents control group event rates of 0.17% and 0.2% in the studies for maternal death, and 2.66% and 1.6% in the studies for severe morbidity. Some studies did not report a measure of variation; based on the large number of participants, we would expect a degree of variation.
1Al‐beity 2019 and Hanson 2021 measured adherence at 10 months' post‐intervention. 2One NRSI reported no difference in PPH ≥ 500 mL (very low certainty due to downgrading by two levels for serious risk of bias and one level for inconsistency, as this result is not in concordance with results from RCTs) (Sloan 2005). 3One NRSI found a reduction in blood transfusions in implementation facilities (odds ratio 0.56, 95% CI 0.48 to 0.65). This is rated very low certainty due to downgrading by two levels for serious risk of bias and one level for inconsistency, as this result is not in concordance with results from RCTs (Mogilevkina 2022). 4Two NRSIs found no difference in mortality (low certainty due to downgrading by two levels for serious risk of bias) (Mogilevkina 2022; Sloan 2005).
Summary of findings 3. Multicomponent implementation strategies versus enhanced usual care for PPH prevention, detection, and treatment.
|
Patients or population: pregnant people admitted to healthcare facility for birth and birth attendants Settings: hospital or healthcare facility Intervention: multicomponent implementation strategy Comparison: enhanced usual care | ||||||
| Outcome | No. of studies | Description of results | Summary of implementation strategy domains used | No. of participants or births (studies, clusters) | Certainty of the evidence (GRADE) | Summary statement |
| Adherence to WHO PPH prevention recommendation during the study period1,2 | 2 | 2 RCTs (Althabe 2008; Evans 2018) reported an increase in adherence to prophylactic uterotonic:
Althabe 2008 reported an increase to another recommendation (P2, Table 2), lowering the episiotomy rate (difference in rate change −10.9, 95% CI −16.1 to −5.8). |
|
14,718 births1 (2 studies, 31 clusters) |
LOWa,b | Multicomponent implementation strategies may improve adherence to WHO PPH prevention recommendations compared to enhanced usual care. |
| Adherence to WHO PPH treatment recommendation during the study period3 | 2 | Deneux‐Tharaux 2010 reported mixed findings (Table 2):
Gallos 2024 reported an increase in adherence to 2 recommendations (T1, Table 2):
|
|
356,913 births (2 studies, 186 clusters) | LOWc,d | Multicomponent implementation strategies may improve adherence to WHO PPH treatment recommendations compared to enhanced usual care. |
| PPH ≥ 500 mL within 24 hours after birth | 4 | 3 RCTs showed a decrease: Gallos 2024 (RR 0.51, 95% CI 0.44 to 0.60); Althabe 2008 (RR 0.55, 95% CI 0.29 to 0.91); Evans 2018 (17% reduction). Deneux‐Tharaux 2010 demonstrated no difference (OR 1.01, 95% CI 0.80 to 1.30). |
|
371,631 births1 (4 studies, 217 clusters) | VERY LOWf,g,4 | We do not know if multicomponent implementation strategies have any effect on PPH ≥ 500 mL compared to enhanced usual care. |
| PPH ≥ 1000 mL within 24 hours after birth | 3 | 3 RCTs reported this outcome. 2 found a reduction: Gallos 2024: RR 0.39, 95% CI 0.31 to 0.49; Althabe 2008: ratio of median relative risk 0.30, 95% CI 0.22 to 0.84. Deneux‐Tharaux 2010 found no difference (OR 1.02, 95% CI 0.83 to 1.24). |
|
371,631 births (3 studies, 205 clusters) | VERY LOWf,h,5 | We do not know if multicomponent implementation strategies have any effect on PPH ≥ 1000 mL compared to enhanced usual care. |
| Additional uterotonics within 24 hours after birth | No studies reported this outcome. | |||||
| Blood transfusion during hospitalization for birth | 2 | Deneux‐Tharaux 2010 reported no difference (OR 1.13, 95% CI 0.88 to 1.44), whereas Gallos 2024 reported a benefit (RR 0.71, 95% CI 0.55 to 0.90). |
|
356,913 births (2 studies, 186 clusters) | VERY LOWc,f,6 | We do not know if multicomponent implementation strategies have any effect on blood transfusion compared to enhanced usual care. |
| Maternal death up until discharge for birth hospitalization and up to 42 days after birth | 2 | 2 RCTs reported this outcome. Althabe 2008 found no difference in mortality:
Gallos 2024 also found no difference, as demonstrated by the absolute risk difference:
|
|
224,850 births (2 studies, 99 clusters) |
MODERATEe,i | Multicomponent implementation strategies probably have little or no effect on maternal death compared to enhanced usual care. |
| Severe morbidity (outcomes related to ICU admission) during hospitalization for birth | 2 | 2 RCTs reported this outcome. Gallos 2024 found no difference between implementation [I] and control [C] arms, as demonstrated by the absolute risk difference: [I] 7 admissions/49,101 women vs [C] 32 admissions/50,558 women; RR 0.70, 95% CI 0.12 to 4.05. Althabe 2008 also found no difference between arms:
|
|
224,850 births (2 studies, 99 clusters) |
MODERATEe,i | Multicomponent implementation strategies probably have little to no effect on severe morbidity (ICU admission) compared to enhanced usual care. |
| Severe morbidity (surgical outcomes) during hospitalization for birth | 1 | Gallos 2024 reported little or no difference in severe morbidity. The RR shows a difference with very wide CIs (RR 1.72, 95% CI 0.57 to 5.16); the absolute risk ranges from 7/50,558 (0.01%) in the control arm vs 12/49,101 (0.02%) in the implementation arm. |
|
210,132 births (1 study, 80 clusters) | MODERATEi,j,7 | Multicomponent implementation strategies probably have little to no effect on severe morbidity (surgical outcomes) compared to enhanced usual care. |
| Adverse effects (variable and related to the intervention) during hospitalization for birth | No studies reported this outcome. | |||||
| CI: confidence interval; ICU: intensive care unit; NRSI: non‐randomized study of interventions; OR: odds ratio; PPH: postpartum hemorrhage; RCT: randomized controlled trial; RR: risk ratio; WHO: World Health Organization. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for imprecision, as some studies did not report measures of variation. The absence of CIs for the analyses available from the studies means that we were not able to assess the CIs for individual studies. Additionally, part of our decision not to proceed with a meta‐analysis was due to different methods of how results, including measures of variation, were presented by the studies. We are therefore unable to draw any meaningful inferences about the precision of estimated effects across studies, and have decided to reflect this uncertainty in our downgrading decision for the effect. bDowngraded one level for risk of bias. We assessed one trial as at high risk of bias (Evans 2018), and the other trial as some concerns (Althabe 2008). cDowngraded one level for risk of bias. We assessed one trial as at high risk of bias (Deneux‐Tharaux 2010), and the other trial as at low risk of bias (Gallos 2024). dDowngraded one level for inconsistency due to differences in results. eDowngraded one level for risk of bias, as Althabe 2008 had some concerns. fDowngraded two levels for inconsistency due to differences in results. gDowngraded one level for risk of bias. We assessed one trial as some concerns (Althabe 2008), and one trial as at high risk of bias (Evans 2018). Another trial was also at overall high risk of bias (Deneux‐Tharaux 2010); however, as it reported no difference between groups for this outcome, we do not expect bias to have influenced the result. hDowngraded one level for risk of bias. We assessed one trial as some concerns (Althabe 2008). We assessed another trial as at overall high risk of bias (Deneux‐Tharaux 2010); however, as it reported no difference between groups for this outcome, we do not expect bias to have influenced the result. iNot downgraded for imprecision; one study reported a wide CI, but this outcome is very rare. jDowngraded one level for indirectness; one study reporting on a very rare outcome, and results may not be generalizable.
1Evans 2018 did not report the number of births in the study cohort. 2Althabe 2008 measured adherence at 12 and 18 months' post‐intervention. Evans 2018 measured adherence at 13 to 16 months' post‐intervention and 21 to 23 months' post‐intervention. 3Deneux‐Tharaux 2010 measured adherence at 12 months' post‐intervention. Gallos 2024 measured adherence at 7 months' post‐intervention. 4One NRSI found an increase in PPH ≥ 500 mL (very low certainty due to downgrading by two levels for serious risk of bias and one level for inconsistency, as this result is not in concordance with results from RCTs) (Main 2017). 5One NRSI found no effect on PPH ≥ 1000 mL (very low certainty due to downgrading by two levels for serious risk of bias and one level for inconsistency, as this result is not in concordance with results from RCTs) (Liabsuetrakul 2017). 6One NRSI found a lower rate of patients requiring blood transfusions in hospitals in the implementation group (very low certainty due to downgrading by two levels for serious risk of bias and one level for inconsistency, as this result is not in concordance with results from RCTs) (Liabsuetrakul 2017). 7One NRSI found a decrease in severe morbidity in the implementation group compared to control (very low certainty due to downgrading by two levels for serious risk of bias and one level for inconsistency, as this result is not in concordance with results from RCTs) (Main 2017).
Background
Although World Health Organization (WHO) guidelines target the prevention, detection, and treatment of postpartum hemorrhage (PPH), a leading cause of maternal mortality worldwide, the implementation of these evidence‐based practices remains suboptimal with maternal mortality and morbidity remaining high.
Description of the condition
PPH is a leading cause of maternal mortality worldwide, accounting for about 27% of maternal deaths [1]. While PPH is relatively common—affecting approximately 14 million births worldwide [2]—approximately 80% of PPH‐related deaths occur in low‐ and middle‐income countries (LMICs), primarily in sub‐Saharan Africa and South Asia, and are thought to be preventable [3, 4, 5, 6]. Reducing the burden of PPH has important health and equity implications toward achieving the Sustainable Development Goal targets [2].
Despite numerous systematic reviews, professional organization reports, and WHO guidelines that describe the effective clinical interventions for preventing, detecting, and treating PPH, variable implementation of these evidence‐based practices has led to slow improvements in health outcomes [2]. The WHO commissioned this review to evaluate effective implementation strategies for incorporating WHO guidelines on PPH prevention, detection, and treatment into practice within birthing facilities.
Description of the intervention and how it might work
Implementation strategies aim to increase the adoption, uptake, implementation, and sustained delivery of evidence‐based practices. In 2015, the Expert Recommendations for Implementing Change (ERIC) developed consensus and clarity around implementation strategy terms and definitions [7]. Examples of common implementation strategies include audit and feedback, educational meetings, simulation, in‐house training, reminders, and targeted financial incentives, among others. Since ERIC, researchers have identified over 70 well‐researched strategies and mapped those into nine discrete categories: 1) Engage consumers; 2) Use evaluative and iterative strategies; 3) Change infrastructure; 4) Adapt and tailor to the context; 5) Develop stakeholder relationships; 6) Utilize financial strategies; 7) Support clinicians; 8) Provide interactive assistance; and 9) Train and educate stakeholders [8, 9].
Broader health system challenges, such as workforce availability, financing, and availability of essential medications, limit high‐quality care related to PPH prevention, detection, and treatment [10]. Implementation strategies can target one or multiple levels (individual healthcare professionals, teams, organizations, systems) and are designed to address barriers or factors that impede healthcare delivery or promote facilitators in specific contexts [11]. Importantly, implementation strategies often require local adaptation for context‐specific barriers that may vary across different settings [12]. Single or multiple implementation strategies may be used to increase adherence to evidence‐based guidelines, which should result in reduced maternal mortality and morbidity.
In 2023, the WHO convened a Guideline Development Group to appraise the evidence for effective clinical interventions as part of a global initiative around PPH [2].Establishing consensus on the most effective practices is a critical first step; integrating these practices into real‐world clinical environments is vital in lowering mortality.
Why it is important to do this review
The effectiveness of individual WHO recommendations related to PPH prevention, detection, and treatment has been reviewed separately (Table 2). Despite clinical guidelines for the prevention, detection, and treatment of PPH, effective implementation has lagged; there is a lack of consensus on the most effective strategies that are responsive to local needs, priorities, and barriers. A reliable evidence review of implementation strategies for PPH prevention, detection, and treatment was needed.
Objectives
To evaluate the clinical benefits and harms of implementation strategies used to promote adherence to WHO clinical guidelines for the prevention, detection, and treatment of postpartum hemorrhage.
Methods
We followed the Methodological Expectations for Cochrane Intervention Reviews (MECIR) when conducting the review, and PRISMA 2020 and Template for Intervention Description and Replication (TIDieR) for the reporting interventions and results [13, 14, 15, 16]. The review protocol was registered on PROSPERO (CRD42024563802).
Differences between protocol and review
We made minor amendments to the PROSPERO‐registered protocol in the reporting of two critical outcomes: (1) adherence to WHO‐recommended practices; and (2) severe morbidity. In this review, we stratified 'adherence to WHO‐recommended practices' by adherence to prevention guidelines (prophylactic oxytocin and episiotomy avoidance) and detection and treatment guidelines (intravenous (IV) oxytocin and uterine massage). In the protocol, 'severe morbidity' was a composite outcome, defined as maternal deaths or severe morbidity events adapted from WHO “near miss” criteria to include major surgery (laparotomy, uterine artery ligation, internal iliac artery ligation, B‐Lynch suture, hysterectomy, extensive vaginal repair), admission to the intensive care unit (ICU), or vital organ failure (temporary or permanent) during hospitalization for birth [17]. For simplified reporting in this review, we disaggregated severe morbidity with ICU admission and severe morbidity requiring major surgery.
Additionally, our registered protocol stated we would review "common implementation strategies." However, we broadened the scope of the review to include all implementation strategies regardless of their prevalence.
We also required cohort studies to have a concurrent control group to create consistent inclusion criteria between study types.
Criteria for considering studies for this review
We excluded studies that had no obtainable, relevant, or interpretable data. We defined ‘relevant data’ as a change in the adherence to a WHO‐recommended PPH prevention, detection, or treatment guideline.
We considered three important comparisons for this review:
single‐component implementation strategies versus usual care;
multicomponent implementation strategies versus usual care;
multicomponent implementation strategies versus enhanced usual care.
We defined 'enhanced usual care' as circumstances where a control population received additional support. This was typically operationalized as facilities that received medications or supplies, or both, without training or changes to local protocols, such as permitting providers at all sites to administer pertinent medications or treatments where there were previously restrictions.
Types of studies
We included randomized controlled trials (RCTs) (cluster, pragmatic, and stepped‐wedge), non‐randomized studies of interventions (NRSI) (interrupted time series (ITS) studies, controlled before‐after (CBA) studies, and follow‐up cohort studies) with concurrent controls. We required ITS studies to have a defined intervention point and two data points before and after the intervention. We also required CBA studies to have contemporaneous data collection and at least one intervention and control site.
We excluded articles published only as abstracts, given that implementation approaches would not be sufficiently described. We included studies regardless of the language of publication.
Although a mixed‐methods review would be ideal, the timeline for the report did not permit this. A recent qualitative synthesis by Akter and colleagues is a useful reference to understand community, women, and clinicians' experience with PPH prevention, detection, and management [18].
Types of participants
This review focused on obstetric healthcare providers and the pregnant or birthing people they care for. Examples of obstetric healthcare providers include doctors, midwives, nurses, and skilled birth attendants. We included studies where people gave birth (vaginal or cesarean) in hospitals or healthcare facilities (i.e. facility‐based childbirth).
We aimed to include studies from any country or region of the world.
Types of interventions
The effectiveness of individual WHO clinical guidelines related to PPH prevention, detection, and treatment was reviewed separately for the WHO Guideline Review Committee (GRC). The focus of this Cochrane review was on implementation strategies for one or more of the recommended practices outlined in the WHO guidelines for PPH management to effect change in the behavior of healthcare professionals and to improve patient outcomes. See Table 2.
To avoid confusion related to the term 'intervention,' we refer to 'implementation strategies' throughout this review. We utilized the ERIC taxonomy to categorize implementation strategies [7, 9]. See Table 5. We described the clinical interventions being implemented according to TIDieR [13]. We included studies that compared a single‐component or multicomponent implementation strategies to usual care. Some studies that utilized multicomponent implementation strategies provided partial support to the control arm, which was denoted as 'enhanced usual care.'
2. ERIC concept mapping and discrete implementation strategy compilation*.
| Domain | Strategy | Definitions |
| Adapt and tailor to context | Promote adaptability | Identify the ways a clinical innovation can be tailored to meet local needs and clarify which elements of the innovation must be maintained to preserve fidelity |
| Tailor strategies | Tailor the implementation strategies to address barriers and leverage facilitators that were identified through earlier data collection | |
| Use data experts | Involve, hire, and/or consult experts to inform management on the use of data generated by implementation efforts | |
| Use data warehousing techniques | Integrate clinical records across facilities and organizations to facilitate implementation across systems | |
| Change infrastructure | Change accreditation or membership requirements | Strive to alter accreditation standards so that they require or encourage use of the clinical innovation. Work to alter membership organization requirements so that those who want to affiliate with the organization are encouraged or required to use the clinical innovation |
| Change liability laws | Participate in liability reform efforts that make clinicians more willing to deliver the clinical innovation | |
| Change physical structure and equipment | Evaluate current configurations and adapt, as needed, the physical structure and/or equipment (e.g. changing the layout of a room, adding equipment) to best accommodate the targeted innovation | |
| Change record systems | Change records systems to allow better assessment of implementation or clinical outcomes | |
| Change service sites | Change the location of clinical service sites to increase access | |
| Create or change credentialing and/or licensure standards | Create an organization that certifies clinicians in the innovation or encourage an existing organization to do so. Change governmental professional certification or licensure requirements to include delivering the innovation. Work to alter continuing education requirements to shape professional practice toward the innovation | |
| Mandate change | Have leadership declare the priority of the innovation and their determination to have it implemented | |
| Start a dissemination organization | Identify or start a separate organization that is responsible for disseminating the clinical innovation. It could be a for‐profit or nonprofit organization | |
| Develop stakeholder interrelationships | Build a coalition | Recruit and cultivate relationships with partners in the implementation effort |
| Capture and share local knowledge | Capture local knowledge from implementation sites on how implementers and clinicians made something work in their setting and then share it with other sites | |
| Conduct local consensus discussions | Include local providers and other stakeholders in discussions that address whether the chosen problem is important and whether the clinical innovation to address it is appropriate | |
| Develop academic partnerships | Partner with a university or academic unit for the purposes of shared training and bringing research skills to an implementation project | |
| Develop an implementation glossary | Develop and distribute a list of terms describing the innovation, implementation, and stakeholders in the organizational change | |
| Identify and prepare champions | Identify and prepare individuals who dedicate themselves to supporting, marketing, and driving through an implementation, overcoming indifference or resistance that the intervention may provoke in an organization | |
| Identify early adopters | Identify early adopters at the local site to learn from their experiences with the practice innovation | |
| Inform local opinion leaders | Inform providers identified by colleagues as opinion leaders or “educationally influential” about the clinical innovation in the hopes that they will influence colleagues to adopt it | |
| Involve executive boards | Involve existing governing structures (e.g. boards of directors, medical staff boards of governance) in the implementation effort, including the review of data on implementation processes | |
| Model and simulate change | Model or simulate the change that will be implemented prior to implementation | |
| Obtain formal commitments | Obtain written commitments from key partners that state what they will do to implement the innovation | |
| Organize clinician implementation team meetings | Develop and support teams of clinicians who are implementing the innovation and give them protected time to reflect on the implementation effort, share lessons learned, and support one another’s learning | |
| Promote network weaving | Identify and build on existing high‐quality working relationships and networks within and outside the organization, organizational units, teams, etc. to promote information sharing, collaborative problem‐solving, and a shared vision/goal related to implementing the innovation | |
| Recruit, designate, and train for leadership | Recruit, designate, and train leaders for the change effort | |
| Use advisory boards and workgroups | Create and engage a formal group of multiple kinds of stakeholders to provide input and advice on implementation efforts and to elicit recommendations for improvements | |
| Use an implementation advisor | Seek guidance from experts in implementation | |
| Visit other sites | Visit sites where a similar implementation effort has been considered successful | |
| Engage consumers | Increase demand | Attempt to influence the market for the clinical innovation to increase competition intensity and to increase the maturity of the market for the clinical innovation |
| Intervene with patients/consumers to enhance uptake and adherence | Develop strategies with patients to encourage and problem solve around adherence | |
| Involve patients/consumers and family members | Engage or include patients/consumers and families in the implementation effort | |
| Prepare patients/consumers to be active participants | Prepare patients/consumers to be active in their care, to ask questions, and specifically to inquire about care guidelines, the evidence behind clinical decisions, or about available evidence‐supported treatments | |
| Use mass media | Use media to reach large numbers of people to spread the word about the clinical innovation | |
| Provide interactive assistance | Centralize technical assistance | Develop and use a centralized system to deliver technical assistance focused on implementation issues |
| Facilitation | A process of interactive problem‐solving and support that occurs in a context of a recognized need for improvement and a supportive interpersonal relationship | |
| Provide clinical supervision | Provide clinicians with ongoing supervision focusing on the innovation. Provide training for clinical supervisors who will supervise clinicians who provide the innovation | |
| Provide local technical assistance | Implementation issues using local personnel | |
| Support clinicians | Create new clinical teams | Change who serves on the clinical team, adding different disciplines and different skills to make it more likely that the clinical innovation is delivered (or is more successfully delivered) |
| Develop resource‐sharing agreements | Develop partnerships with organizations that have resources needed to implement the innovation | |
| Facilitate relay of clinical data to providers | Provide as close to real‐time data as possible about key measures of process/outcomes using integrated modes/channels of communication in a way that promotes use of the targeted innovation | |
| Remind clinicians | Develop reminder systems designed to help clinicians to recall information and/or prompt them to use the clinical innovation | |
| Revise professional roles | Shift and revise roles among professionals who provide care, and redesign job characteristics | |
| Train and educate stakeholders | Conduct educational meetings | Hold meetings targeted toward different stakeholder groups (e.g. providers, administrators, other organizational stakeholders, and community, patient/consumer, and family stakeholders) to teach them about the clinical innovation |
| Conduct educational outreach visits | Have a trained person meet with providers in their practice settings to educate providers about the clinical innovation with the intent of changing the provider’s practice | |
| Conduct ongoing training | Plan for and conduct training in the clinical innovation in an ongoing way | |
| Create a learning collaborative | Facilitate the formation of groups of providers or provider organizations and foster a collaborative learning environment to improve implementation of the clinical innovation | |
| Develop educational materials | Develop and format manuals, toolkits, and other supporting materials in ways that make it easier for stakeholders to learn about the innovation and for clinicians to learn how to deliver the clinical innovation | |
| Distribute educational materials | Distribute educational materials (including guidelines, manuals, and toolkits) in person, by mail, and/or electronically | |
| Make training dynamic | Vary the information delivery methods to cater to different learning styles and work contexts, and shape the training in the innovation to be interactive | |
| Provide ongoing consultation | Provide ongoing consultation with one or more experts in the strategies used to support implementing the innovation | |
| Shadow other experts | Provide ways for key individuals to directly observe experienced people engage with or use the targeted practice change/innovation | |
| Use train‐the‐trainer strategies | Train designated clinicians or organizations to train others in the clinical innovation | |
| Work with educational institutions | Encourage educational institutions to train clinicians in the innovation | |
| Use evaluative and iterative strategies | Assess for readiness and identify barriers and facilitators | Assess various aspects of an organization to determine its degree of readiness to implement, barriers that may impede implementation, and strengths that can be used in the implementation effort |
| Audit and provide feedback | Collect and summarize clinical performance data over a specified time period and give it to clinicians and administrators to monitor, evaluate, and modify provider behavior | |
| Conduct cyclical small tests of change | Implement changes in a cyclical fashion using small tests of change before making changes system‐wide. Tests of change benefit from systematic measurement, and results of the tests of change are studied for insights on how to do better. This process continues serially over time, and refinement is added with each cycle | |
| Conduct local needs assessment | Collect and analyze data related to the need for the innovation | |
| Develop a formal implementation blueprint | Develop a formal implementation blueprint that includes all goals and strategies. The blueprint should include the following: 1) aim/purpose of the implementation; 2) scope of the change (e.g. what organizational units are affected); 3) timeframe and milestones; and 4) appropriate performance/progress measures. Use and update this plan to guide the implementation effort over time | |
| Develop and implement tools for quality monitoring | Develop, test, and introduce into quality‐monitoring systems the right input—the appropriate language, protocols, algorithms, standards, and measures (of processes, patient/consumer outcomes, and implementation outcomes) that are often specific to the innovation being implemented | |
| Develop and organize quality monitoring systems | Develop and organize systems and procedures that monitor clinical processes and/or outcomes for the purpose of quality assurance and improvement | |
| Obtain and use patients/consumers and family feedback | Develop strategies to increase patient/consumer and family feedback on the implementation effort | |
| Purposely re‐examine the implementation | Monitor progress and adjust clinical practices and implementation strategies to continuously improve the quality of care | |
| Stage implementation scale‐up | Phase implementation efforts by starting with small pilots or demonstration projects and gradually move to a system‐wide rollout | |
| Utilize financial strategies | Access new funding | Access new or existing money to facilitate the implementation |
| Alter incentive/allowance structures | Work to incentivize the adoption and implementation of the clinical innovation | |
| Alter patient/consumer fees | Create fee structures where patients/consumers pay less for preferred treatments (the clinical innovation) and more for less‐preferred treatments | |
| Develop disincentives | Provide financial disincentives for failure to implement or use the clinical innovations | |
| Fund and contract for the clinical innovation | Governments and other payers of services issue requests for proposals to deliver the innovation, use contracting processes to motivate providers to deliver the clinical innovation, and develop new funding formulas that make it more likely that providers will deliver the innovation | |
| Make billing easier | Make it easier to bill for the clinical innovation | |
| Place innovation on fee for service lists/formularies | Work to place the clinical innovation on lists of actions for which providers can be reimbursed (e.g. a drug is placed on a formulary, a procedure is now reimbursable) | |
| Use capitated payments | Pay providers or care systems a set amount per patient/consumer for delivering clinical care | |
| Use other payment schemes | Introduce payment approaches (in a catch‐all category) |
ERIC: Expert Recommendations for Implementing Change
*Table adapted from [9].
Outcome measures
To develop the list of priority outcomes, we evaluated the PPH core outcome set and lists of critical and important outcomes from previous WHO PPH guidelines. To be eligible for inclusion in the review, studies must have reported both a clinical and implementation outcome. Simply assessing implementation approaches without clinical outcomes would not help inform decision‐makers whether the strategies are impactful.
We used processes described by the COMET Initiative [19], gaining consensus among all review team members, WHO policy leaders, and Cochrane methodologists to prioritize critical and important outcomes.
Critical outcomes
Adherence to WHO‐recommended PPH prevention clinical guidelines, operationalized as the extent to which providers gave and/or patients received recommended therapies at birth (prophylactic oxytocin and avoidance of episiotomy).
Adherence to WHO‐recommended PPH detection and treatment clinical guidelines, operationalized as the extent to which providers gave and/or patients received recommended treatments (e.g. oxytocin, sulprostone, misoprostol, tranexamic acid) within the intrapartum and immediate postpartum periods, within 24 hours of birth and by discharge. Guideline‐specific WHO parameters were also abstracted.
PPH ≥ 500 mL within 24 hours after birth.
PPH ≥ 1000 mL within 24 hours after birth.
Additional uterotonics within 24 hours after birth.
Blood transfusion during hospitalization for birth.
Maternal death up until discharge for birth hospitalization and up to 42 days after birth.
Severe morbidity, defined as maternal deaths or severe morbidity events adapted from WHO “near miss” criteria, including admission to the ICU or vital organ failure (temporary or permanent) during hospitalization for birth [17].
Severe morbidity, defined as maternal deaths or severe morbidity events adapted from WHO “near miss” criteria, including major surgery (laparotomy, uterine artery ligation, internal iliac artery ligation, B‐Lynch suture, hysterectomy, extensive vaginal repair) or vital organ failure(temporary or permanent) during hospitalization for birth [17].
Adverse effects (variable and related to the implementation strategy) during hospitalization for birth.
Important outcomes
Breastfeeding at discharge.
-
Implementation factors: implementation outcomes are adopted from the framework presented by [20, 21], and vary depending on whether the implementation strategy was designed for PPH prevention, detection, or management [13, 20]:
Acceptability of the implementation strategy: satisfaction with various aspects of the implementation strategy
Adoption of the implementation strategy: uptake, utilization, intention to try
Appropriateness of the implementation strategy: compatibility, usefulness, perceived fit
Feasibility of the implementation strategy: practicality
Fidelity of the implementation strategy: delivered as intended, adherence, integrity
Implementation cost of the implementation strategy: cost‐effectiveness, cost‐benefit
Penetration of the implementation strategy: degree of institutionalization
Sustainability of the implementation strategy: continuation, durability, institutionalization, sustained use, routinization
-
Healthcare professional outcomes:
Changes in knowledge, skills
Search methods for identification of studies
Electronic searches
We searched the following databases on 25 April 2024:
Cochrane Register of Controlled Trials (CENTRAL) (2024, Issue 4);
MEDLINE (Ovid SP) 1946 to 25 April 2024;
Embase (Ovid SP) 1980 to 2024 week 17;
CINAHL (EBSCOhost) (Cumulative Index to Nursing and Allied Health Literature) 1981 to 25 April 2024;
ClinicalTrials.gov (clinicaltrials.gov) (2000 to 25 April 2024);
WHO International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/Default.aspx) (1990 to 25 April 2024).
See Supplementary material 1 for details of the sources searched and the search strategies for each source.
Post‐publication amendments and retractions were included in the full search. We did not apply any language or date restrictions, or filters or automation to the search.
Searching other resources
We examined the reference lists of recently published systematic reviews [18, 22].
Data collection and analysis
Selection of studies
To ensure consistent application of inclusion criteria, a pilot was conducted where the authors responsible for extraction reviewed 20 articles for title/abstract screening. We reviewed and discussed the results and modified the screening tool. Two review authors (from EL, RM, MMD, KS) independently assessed the title, abstract, and full text of all the potential studies identified for inclusion. Any disagreements were resolved through discussion and in consultation with a third review author (JMG).
We excluded studies with no relevant and interpretable data presented or obtainable; we defined ‘relevant data’ as a change in the adherence of a PPH management guideline. RCTs, NRSI, and CBA studies had to have sufficient data to estimate effect size in at least one relevant outcome after implementation. ITS studies must have included clearly defined implementation points. See also Types of studies.
We used Covidence to screen titles, abstracts, and full‐text reports [23]. We documented the study selection process in a PRISMA flow diagram, including reasons for exclusion of full‐text studies initially considered to be potentially relevant. When studies required translation into English for consideration, we contacted the Cochrane Community for translation support and used a standardized Microsoft Word document for translation [24].
Data extraction and management
Two review authors (from RM, KS, LC, LR) independently extracted data from the included studies using a blank electronic form (Covidence for implementation strategies and study‐level information; Microsoft Excel for outcomes data) [25]. Any disagreements about data extraction were resolved through discussion.
We extracted the following information on study characteristics.
Number and characteristics of patient and provider participants
Country, clinical setting (urban, rural, in‐hospital) and level of facility (primary, secondary, tertiary)
Study design, inclusion and exclusion criteria
Evidence‐based recommendation(s) being implemented
Targeted behavior change (management and relevant WHO recommendation)
Details on comparison arm (supplies, staffing, etc.)
Implementation strategy (including theoretical underpinnings, implementation strategy components, mode of delivery, frequency, intensity, duration, tailoring)
Health outcomes (as listed in Outcome measures)
We extracted the following outcome data.
Number of participants
Any exclusion criteria
Implementation strategies being compared and their respective primary and secondary outcomes
Relevant arm‐level data (e.g. number of events and number of participants for binary outcomes and means and standard deviations (SDs) per study arm for continuous outcomes)
Harms
Costs and/or resources required
Data on potential effect modifiers
We extracted the following study, clinical intervention, and population characteristics that may act as effect modifiers.
Mode of birth (vaginal or cesarean birth)
Prior risk of PPH (as defined by trialists and categorized as low, high, mixed or not stated)
Other data
We extracted the following additional information.
Date of publication and dates of recruitment
Type of publication (full‐text reports, abstracts, unpublished data)
Trial registration reference
When information was unclear, we contacted the authors of the original reports via email to request further details.
Risk of bias assessment in included studies
Randomized controlled trials (RCTs)
Two review authors (from EL, MMD, LC, LR, JMG) independently assessed the risk of bias in randomized trials using the Excel tool for the Cochrane RoB 2 tool [26]. We resolved any disagreement by discussion or by involving a third review author. We were interested in the effect of assigning participants to the implementation arm. Consequently, we conducted analyses based on the intention‐to‐treat (ITT) principle, which includes all randomized participants regardless of the implementation strategy or clinical guideline actually received.
For each critical outcome reported by an included trial, we assessed the following risk of bias domains.
(1a) Bias arising from the randomization process
(1b) (For cluster‐randomized trials only) Bias arising from identification or recruitment of individual participants within clusters
(2) Bias due to deviations from intended implementation strategies
(3) Bias due to missing outcome data
(4) Bias in measurement of the outcome
(5) Bias in selection of the reported result
(6) Overall bias
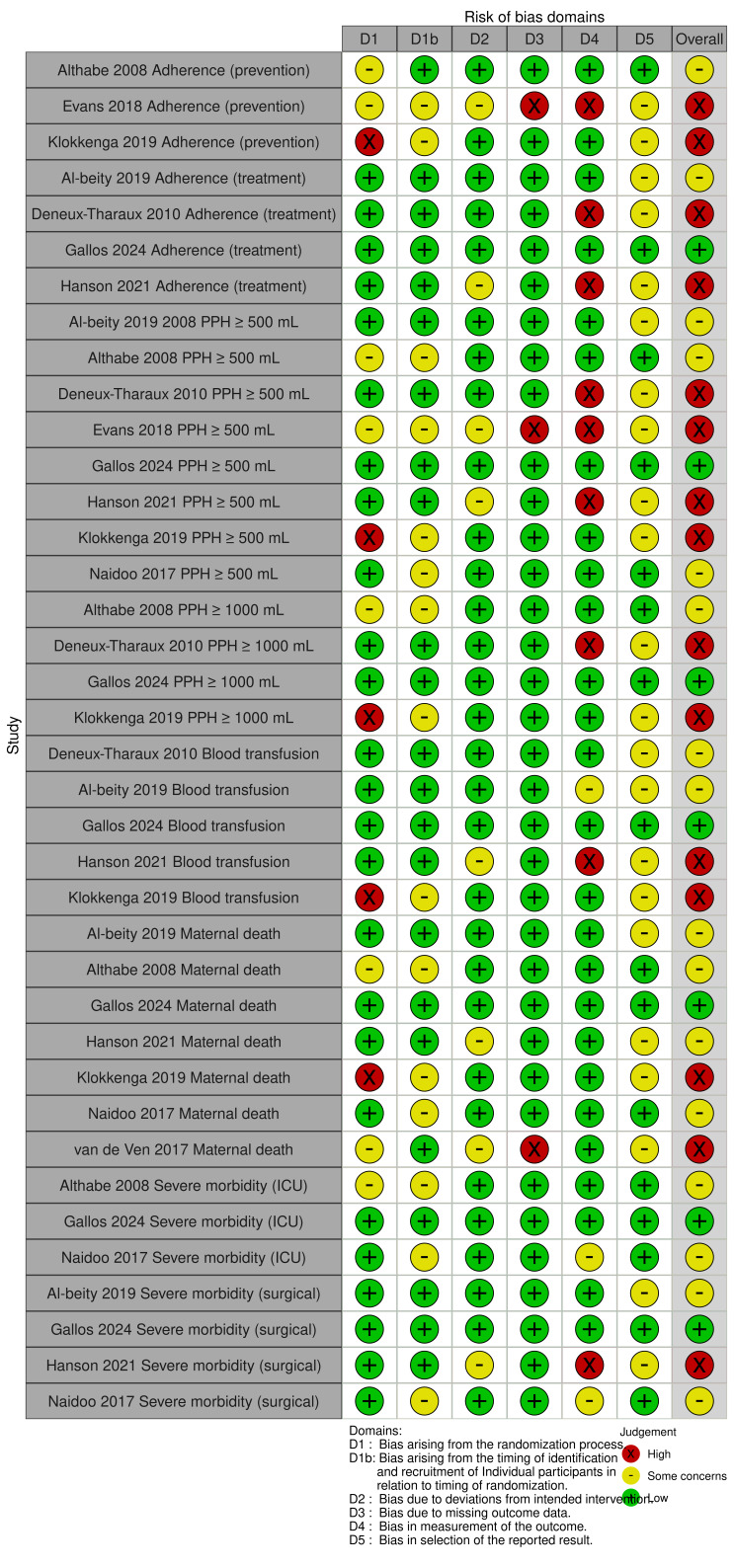
For each domain listed above, we provided an explicit assessment of whether the study was at low risk, some concerns, or high risk of bias. During assessment, we recorded any subjective judgments, important concerns about the methods, and potential sources of bias. We also assessed the likely magnitude and direction of the bias and whether it was considered likely to impact on the findings. For the final assessment of overall risk of bias, we made explicit judgments about whether studies were at high risk of bias, according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions [27]. We used robvis (visualization tool) [28] to generate Figure 1 and Figure 2.
1.
RoB 2 assessments for cluster‐RCTs.
2.
ROBINS‐I assessments for non‐randomized studies.
In addition, we used a research integrity assessment tool to establish the integrity and authenticity of studies, the Cochrane Trustworthiness Screening Tool, developed by Cochrane Pregnancy and Childbirth [29]. See Figure 3.
3.

Trustworthiness Tool.
Screening eligible studies for scientific integrity/trustworthiness
Two review authors (from RM, KS, LC, LR) evaluated all eligible studies against predefined criteria to select studies that, based on available information, were deemed to be sufficiently trustworthy to be included in the analysis.
When we had questions about study trustworthiness, we contacted the authors via email. If we were still unable to obtain adequate information, we categorized the study as awaiting classification and described the concerns and communications with the author (or lack thereof) in detail. The process is described fully in Figure 3. The evaluation criteria were as follows.
Research governance
No prospective trial registration for studies published after 2010 without plausible explanation.
When requested, the trial authors refuse to provide/share the protocol or ethics approval letter (or both).
Trial authors refuse to engage in communication with the Cochrane review authors.
Trial authors refuse to provide trial data upon request with no justifiable reason.
Baseline characteristics
Characteristics of the study participants being too similar (distribution of mean (SD) excessively narrow or excessively wide).
Feasibility
Implausible numbers (e.g. 500 women with severe cholestasis of pregnancy recruited in 12 months).
(Close to) zero losses to follow‐up without plausible explanation.
Results
Implausible results (e.g. massive risk reduction for main outcomes with small sample size).
Unexpectedly even numbers of women 'randomized,' including a mismatch between the numbers and the methods, e.g. if it is stated that no blocking was used, but there are still equal numbers, or it is stated that blocks of four were used, but the final numbers differ by six.
Non‐randomized studies of interventions (NRSI)
We assessed risk of bias in NRSI using the Risk Of Bias In Non‐randomized Studies ‐ of Interventions (ROBINS‐I) tool per the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [27]. We assessed risk of bias in relation to the effect of assignment.
For each critical outcome reported by an included study, we assessed the following risk of bias domains:
confounding;
selection of participants into the study;
classification of interventions;
deviation from intended interventions;
missing data;
measurement of outcomes; and
selection of reported result.
Responses to signaling questions contribute to the determination of domain‐specific risk of bias assessments, categorized as no information, low, moderate, serious, or critical risk of bias. For assessment of confounding, we considered 'medical risk women served by the facility' and 'readiness of a health facility to implement clinical guidelines, including the facility structure and supply availability' as confounding domains. We did not consider any important care enhancements that may impact bias. These domain‐specific assessments then inform the overall risk of bias judgment.
Full responses to signaling questions regarding the risk of bias assessment are available upon request.
Measures of treatment effect
Continuous data
For continuous data, we planned to use mean differences (MDs) and SDs to report outcomes.
Dichotomous data
For dichotomous data, we planned to report risk ratios (RRs) with 95% confidence intervals (CIs) for binary outcomes. For time‐to‐event data, we planned to report hazard ratios (HRs) and 95% CIs.
Outcome categories included continuous and dichotomous measures of adherence to WHO‐recommended practices, patient outcomes, and clinician knowledge. We included all outcomes of the studies if they were outcomes of this review. We also collected the timing of data collection and the method for measurement when appropriate. In particular, for the estimation of blood loss and definition of PPH, we extracted the methodology of that measurement (see Table 6). If it was possible to conduct meta‐analysis, we planned to provide MDs and SDs for the continuous measures of estimation of blood loss.
3. Definitions of postpartum hemorrhage by study*.
| Study name | Measurement method | Measurement device | PPH ≥ 500 mL | PPH ≥ 1000 mL | Other definition |
| Althabe 2008 | Volumetric | Collection drape | X | X | |
| Al‐beity 2019 | Visual | Collection drape, soaked cloths | ‐ | ‐ | |
| Deneux‐Tharaux 2010 | Clinical | ‐ | ‐ | ‐ | PPH: decrease in baseline Hb ≥ 2 g/dL Severe PPH: decrease in baseline Hb ≥ 4 g/dL |
| Evans 2018 | Visual | ‐ | X | ‐ | |
| Gallos 2024 | Volumetric | Collection drape | X | X | |
| Hanson 2021 | ‐ | ‐ | ‐ | X | |
| Klokkenga 2019 | Volumetric | Collection drape | X | X | |
| van de Ven 2017 | ‐ | ‐ | ‐ | ‐ | Severe PPH: > 4 packed cells blood transfusion, embolization or hysterectomy |
| Main 2017 | Volumetric | ‐ | X | ‐ | Obstetric hemorrhage was defined as parturients with ICD versions 9 and 10 diagnosis code for antepartum or postpartum hemorrhage, placenta previa, abruptio placentae, or the procedure code for transfusion. |
| Mogilevkina 2022 | Volumetric | Absorbent pads | ‐ | X | |
| Liabsuetrakul 2017 | ‐ | ‐ | X | X | |
| Sloan 2005 | ‐ | ‐ | ‐ | ‐ | |
| Naidoo 2017 | ‐ | ‐ | ‐ | ‐ | Undefined, based on patient record |
Hb: hemoglobin; ICD: International Statistical Classification of Diseases; PPH: postpartum hemorrhage
*Note these definitions are as the study defined postpartum hemorrhage and are not related to clinical outcomes abstracted/assessed in the systematic review.
Unit of analysis issues
We included cluster‐randomized trials in the analyses along with individually randomized trials. We planned to adjust study sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions [27], employing an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we used ICCs from other sources, we would report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We planned to synthesize the relevant information if we identified both cluster‐ and individually randomized trials.
We considered it reasonable to combine the results from both cluster‐ and individually randomized trials if there was little heterogeneity between study designs, and interaction between the effect of the implementation strategy and the choice of randomization unit was considered to be unlikely. However, given the significant heterogeneity of the included studies, we were unable to combine study results.
We had planned to combine implementation groups to avoid double‐counting participants for multi‐arm studies when used in meta‐analysis; however, we did not apply this method because we did not conduct any quantitative synthesis.
Dealing with missing data
We contacted authors to seek clarification when necessary for descriptions of clinical guideline implementation, trial conduct, and availability of unpublished outcome data. We received no responses from study authors. We addressed risk of bias due to incomplete outcome data through use of the Cochrane RoB 2 tool [26].
Reporting bias assessment
We planned to investigate reporting biases using funnel plots if there were 10 or more studies in the meta‐analysis, per the Cochrane Handbook for Systematic Reviews of Interventions [30]. However, given the heterogeneity of the included studies, we were unable to perform any meta‐analyses. In future updates, where feasible, we will investigate reporting biases (such as publication bias) by visually assessing funnel plot asymmetry.
Synthesis methods
We planned to perform standard pair‐wise meta‐analyses using a random‐effects model for every comparison with at least two trials, using RevMan software [31]. However, meta‐analysis was precluded by the lack of included studies or consistency of outcome measurement approach across studies. We tabulated study characteristics and grouped studies based on their implementation strategies. We synthesized findings based on three comparisons:
single‐component implementation strategies versus usual care;
multicomponent implementation strategies versus usual care;
multicomponent implementation strategies versus enhanced usual care.
Investigation of heterogeneity and subgroup analysis
We planned to assess statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. In particular, we planned to carry out a subgroup analysis based on the mode of birth (vaginal birth, cesarean birth, all). We extracted this information from studies and included it in an 'Overview of included studies and syntheses' table (Table 7). We also investigated clinical and methodological heterogeneity, which were addressed through the construction of our comparison groups and explored in the Discussion section.
4. 'Overview of included studies and syntheses' table, illustrating key characteristics of studies, outcomes, and synthesis, sorted alphabetically.
|
Study (country) |
Study setting |
Implementation strategy domain1 | Number of WHO guidelines addressed | Comparison group | Birthing population | No. of participants (intervention/control) | Critical health outcomes measured | Evidence‐based practices measured | Outcome assessment time points |
| Randomized studies | |||||||||
| Althabe 2008 (Argentina and Uruguay) |
19 public maternity hospitals |
|
5 | Usual care | Vaginal births |
Clusters
|
|
|
|
Birth attendants
| |||||||||
Births
| |||||||||
| Al‐beity 2019 (Tanzania) |
61 district hospitals and health centers |
|
8 | Usual care | All |
Clusters
|
|
Uterotonic medications for treatment of PPH |
|
Facilities
| |||||||||
Birth attendants
| |||||||||
Births
| |||||||||
| Deneux‐Tharaux 2010 (France) | 106 maternity units |
|
5 | Enhanced usual care | All |
Clusters
|
|
Uterotonic medications for treatment of PPH | 12 months post‐intervention |
Birth attendants
| |||||||||
Births
| |||||||||
| Evans 2018 (Uganda) | 125 facilities |
|
11 | Enhanced usual care | All |
Cluster
|
PPH ≥ 500 mL2 | Oxytocin for prevention of PPH |
|
Facilities
| |||||||||
Birth attendants
| |||||||||
Births
| |||||||||
| Gallos 2024 (Kenya, Nigeria, South Africa, Tanzania) |
80 secondary‐level facilities |
|
6 | Enhanced usual care | Vaginal births |
Clusters
|
|
|
7 months post‐intervention |
Birth attendants
| |||||||||
Births
| |||||||||
| Hanson 2021 (Uganda) |
22 hospitals and 21 health centers |
|
8 | Usual care | All |
Clusters
|
|
Uterotonic medications for treatment of PPH |
|
Facilities
| |||||||||
Birth attendants
| |||||||||
Births
| |||||||||
| Klokkenga 2019 (Ghana) |
15 hospitals |
|
5 | Usual care | Vaginal births |
Clusters
|
|
|
9 weeks post‐intervention |
Birth attendants
| |||||||||
Births
| |||||||||
| Naidoo 2017 (South Africa) |
18 public sector hospitals (12 district hospitals, 6 regional hospitals) |
|
1 | Usual care | Cesarean births |
Cluster
|
|
** | 6 months post‐intervention |
Birth attendants
| |||||||||
Births
| |||||||||
| van de Ven 2017 (Netherlands) | 24 obstetric units |
|
2 | Usual care | All |
Clusters
|
Blood transfusion5 | ** | 12 months post‐intervention |
Birth attendants6
| |||||||||
Births
| |||||||||
| Non‐randomized studies | |||||||||
| Main 2017 (United States) |
147 hospitals |
|
10 | Enhanced usual care | All |
Clusters
|
|
** |
|
Birth attendants
| |||||||||
Births
| |||||||||
| Mogilevkina 2022 (Ukraine) | 77 hospitals (44 district hospitals, 33 city hospitals) |
|
14 | Usual care | All |
Clusters
|
|
** |
|
Birth attendants
| |||||||||
Births
| |||||||||
| Liabsuetrakul 2017 (Thailand) | 6 community hospitals |
|
2 | Enhanced usual care | Vaginal |
Clusters
|
|
** |
|
Birth attendants
| |||||||||
Births
| |||||||||
| Sloan 2005 (Vietnam) | 48 hospitals and clinics |
|
6 | Usual care | Vaginal |
Clusters
|
|
** | 15 months post‐intervention |
Facilities
| |||||||||
Birth attendants7
| |||||||||
Births7
| |||||||||
| Awaiting classification | |||||||||
| Jayanna 2016 (India) | 108 primary health centers |
|
NR | Enhanced usual care | NR |
Clusters
|
Undefined; pending author response | ** | 12 months post‐intervention |
Birth attendants
| |||||||||
Births
| |||||||||
| Abbreviations: NR: not reported; PPH: postpartum hemorrhage; WHO: World Health Organization | |||||||||
1Implementation strategy domain as outlined in Waltz and colleagues [9]. 2Postpartum hemorrhage occurred during birth and up to 24 hours postpartum. 3Mortality recorded beginning at hospitalization for birth and until 42 days postpartum. 4Severe morbidity measured during hospitalization for birth and adapted from WHO 'near miss' criteria to include major surgery (laparotomy, uterine artery ligation, internal iliac artery ligation, B‐Lynch suture, hysterectomy, extensive vaginal repair), admission to the intensive care unit, or vital organ failure (temporary or permanent). 5Blood transfusion occurred during hospitalization for birth. 6van de Ven 2017 created 74 multiprofessional teams who received the intervention with a mixture of 74 gynecologists, 36 residents, 79 midwives, and 282 nurses. Birth attendant profession for the control arm was not reported. 7Sloan 2005 had two intervention arms and reported birth attendants (108 hospital only/116 hospital and clinic) and births (782 hospital only/922 hospital and clinic) for each arm.
**Evidence‐based practice measurement not reported.
Equity‐related assessment
Though the incidence of PPH is similar around the world, maternal mortality and severe morbidity due to PPH are concentrated in low‐resource settings and disproportionately affect women who are socially disadvantaged. Recognizing that context may influence the implementation of a clinical guideline, we extracted data on the country or countries in which trials were conducted (as a proxy for resource level) and considered how contextual factors influenced the transferability and applicability of results in our interpretation of the evidence.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effect of trial quality for each comparison by restricting the analysis to those trials assessed as at low risk of bias for random sequence generation and allocation concealment. However, this was not possible due to the lack of studies for meta‐analysis.
Certainty of the evidence assessment
We assessed the certainty of the overall evidence using the GRADE approach [32]. We created summary of findings tables, a summary of the implementation strategy effect, and a measure of certainty for each critical outcome (Critical outcomes), which was produced using GRADEpro GDT software [33]. We assessed and presented evidence for all available comparisons:
single‐component implementation strategies versus usual care;
multicomponent implementation strategies versus usual care;
multicomponent implementation strategies versus enhanced usual care.
We assessed the certainty of the body of evidence for each outcome using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, publication bias, and overall risk of bias), downgrading the certainty of the evidence from 'high' by one level for serious (or by two levels for very serious) limitations.
Two review authors (from EL, MMD, LR, LC) independently assessed the certainty of the evidence. Any disagreements between authors were resolved through discussion or consultation with a third review author (JMG) if necessary. All review authors discussed the GRADE ratings via conference calls. Decisions to downgrade or upgrade the certainty of the evidence are noted in the summary of findings tables.
We assessed the certainty of evidence for each outcome as high, moderate, low, or very low, in accordance with the GRADE approach, as described below.
High certainty: we are very confident that the true effect lies close to that of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Consumer involvement
We did not involve consumers or other interest‐holders in this review due to limited resources.
Results
Description of studies
Results of the search
A PRISMA flow diagram of the screening and selection process is presented in Figure 4. The search yielded 5956 records. After removing duplicates, we screened 4278 records by title and abstract. We excluded 4239 clearly irrelevant records and retrieved the full texts for the remaining 39 records. We excluded 22 of these 39 full‐text articles. After full‐text screening, we included 13 studies for descriptive synthesis (Al‐beity 2019 [34]; Althabe 2008 [35]; Deneux‐Tharaux 2010 [36]; Evans 2018 [37]; Gallos 2024 [38]; Hanson 2021 [39]; Klokkenga 2019 [40]; Liabsuetrakul 2017 [41]; Main 2017 [42]; Mogilevkina 2022 [43]; Naidoo 2017 [44]; Sloan 2005 [45]; van de Ven 2017 [46]). None of the included studies were used for meta‐analysis. We also identified three ongoing studies (ISRCTN17679951 2023 [47]; jRCT1090220328 2018 [48]; Kamala 2021 [49]) and one study awaiting classification (Jayanna 2016 [50]).
4.

PRISMA flow diagram.
See Supplementary material 2; Supplementary material 3; Supplementary material 4; Supplementary material 5.
Included studies
A summary of the 13 included studies is provided in the 'Overview of included studies and syntheses' table (Table 7). Further details are available in Supplementary material 2.
Study designs and settings
We included nine cluster‐RCTs (Al‐beity 2019; Althabe 2008; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Naidoo 2017; van de Ven 2017) and four non‐randomized follow‐up (cohort) studies (Liabsuetrakul 2017; Main 2017; Mogilevkina 2022; Sloan 2005). Six studies took place in Africa (Al‐beity 2019; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Naidoo 2017), three in Europe (Deneux‐Tharaux 2010; Mogilevkina 2022; van de Ven 2017), two in Asia (Liabsuetrakul 2017; Sloan 2005), one in North America (Main 2017), and one in South America (Althabe 2008). The diverse geographies in the included studies precluded direct comparisons by facility type (e.g. primary, secondary, tertiary) due to varying facility definitions across studies. The number of facilities in each study ranged from 6 community hospitals in Thailand to 147 facilities in the United States.
Participants
All studies included birth attendants (e.g. doctors, midwives, nurses) as recipients of the clinical guideline implementation, and collected outcomes from patients who gave birth in study facilities.
Birth attendants
The type of birth attendants included in the studies varied based on cadre, training, and years of experience.
Patients
Seven studies included outcomes of all patients who gave birth at the study facilities during the study period. Five studies only included patients who had a vaginal birth. One study only included patients who had a cesarean birth.
Interventions
All studies used "train and educate stakeholders" as a core implementation strategy (Table 8). Three studies exclusively used "train and educate" (Klokkenga 2019; Naidoo 2017; van de Ven 2017), while the other 10 studies employed an additional one to four implementation strategies. See below for further details.
5. Implementation strategy outcome information for the included studies.
| Study | PPH focused (yes/no) | Implementation strategy domain1 | Implementation strategy1 |
Action target |
Action | Dose | Frequency | Duration | Control arm |
| Randomized studies | |||||||||
| Althabe 2008 | No2 |
|
Audit and provide feedback | Birth attendants (physicians, residents, midwives) | Opinion leaders | NR | Throughout the intervention | 18 months | Standard in‐service training |
| Build a coalition | |||||||||
| Conduct ongoing training | Intensive training |
5‐day session with 1‐day refresher | Once | ||||||
| Conduct local needs assessment | |||||||||
| Distribute educational materials | Monthly regional meeting | Once‐monthly meeting | Throughout the intervention | ||||||
| Provide local technical assistance | |||||||||
| Use data experts | Computerized guidelines/intervention materials | Available computer | Throughout the intervention | ||||||
| Al‐beity 2019 | Yes |
|
Identify & prepare champions | Clinical officer, nurse midwives (enrolled or certified) | 1‐day training HMS BAB | Single‐day program with following weekly drills | Once a week for 6 to 8 weeks 30 to 40 minutes per weekly drill |
2 months | Usual care |
| Remind clinicians | |||||||||
| Use train‐the‐trainer strategies | |||||||||
| Model and simulate change | |||||||||
| Deneux‐Tharaux 2010 | Yes |
|
Audit and provide feedback | Local target clinicians (obstetricians, midwives, and anesthetists) | Obstetrician/midwife peers Educational program by study staff |
2 visits per unit | 2 sessions | 4 months | The protocol for early management of PPH was presented at a staff meeting and passively disseminated. |
| Conduct educational outreach visits | |||||||||
| Conduct local needs assessment | |||||||||
| Develop and organize quality monitoring systems | |||||||||
| Inform local opinion leaders | |||||||||
| Remind clinicians | |||||||||
| Promote network weaving | |||||||||
| Evans 2018 | No2 |
|
Audit and feedback | Frontline providers (nurses, midwives, labor & delivery staff) | OSCE and direct observation | 1 day | 2 x 1‐day trainings | 5‐month intervention period | Received the same 1‐day training as intervention group, but no other support |
| Model and simulate change | Team training | 2 single‐day trainings | At the start of study | ||||||
| Provide clinical supervision | Identification to act as mentors | Once | At the start of study | ||||||
| Use train‐the‐trainer strategies | Ongoing mentorship/training | Weekly | Throughout the study | ||||||
| Gallos 2024 | Yes |
|
Audit and provide feedback | Birth attendants | Audit newsletters | Monthly | Throughout the intervention | 7 months | Usual care Collection drapes without an alert/action lines |
| Change physical structure and equipment | Restocking supplies | Every shift | |||||||
| Calibrated EBL drape | NR | ||||||||
| Conduct ongoing training | ‐ | ‐ | |||||||
| Conduct educational outreach visits | |||||||||
| Inform local opinion leaders | |||||||||
| Model and simulate change | Onsite simulation | 90 min to 8 hours | |||||||
| Tailor strategies | Local midwives/MD | NR | |||||||
| Provide clinical supervision | |||||||||
| Hanson 2021 | Yes |
|
Identify & prepare champions | Doctors, medical clinicians, midwives, and nurses | 1‐day training HMS BAB | Single‐day program with following weekly drills | Once a week for 6 to 8 weeks 30 to 40 minutes per weekly drill |
2 months | Usual care |
| Remind clinicians | |||||||||
| Model and simulate change | |||||||||
| Use train‐the‐trainer strategies | |||||||||
| Klokkenga 2019 | No2 |
|
Distribute educational materials | Midwives | Trained research assistants | Once | 3 days | Single training | Usual care |
| Safe Delivery App | Once | NR | Throughout the study | ||||||
| Naidoo 2017 | No2 |
|
Mandate change | Physicians and RNs | Single session MCSSL | Once | NR | 1 month | Usual care |
| van de Ven 2017 | No2 |
|
Model and simulate change | Frontline providers (obstetricians, gynecologists, midwives, residents, nurses) | Single session | 1 day (8 hours) | NR | 9 months | Usual care |
| Non‐randomized studies | |||||||||
| Main 2017 | Yes |
|
Audit and provide feedback | Birth attendants (physicians, nurses) | Ongoing mentorship | NR | Monthly | Throughout the study | Participation in the rapid‐cycle California Maternal Data Center |
| Capture and share local knowledge | |||||||||
| Change physical structure and equipment | |||||||||
| Conduct cyclical small tests of change | |||||||||
| Develop resource‐sharing agreements | |||||||||
| Make training dynamic | Team training | Full day | NR | At start and end of study | |||||
| Provide ongoing consultation | Audit | Once | Monthly | Throughout the study | |||||
| Mogilevkina 2022 | No2 |
|
Audit and provide feedback | Birth attendants (obstetricians/gynecologists, midwives) | AIP trainings | 8 total sessions | NR | Throughout the study | Usual care |
| Model and simulate change | Pre‐/post‐exams | After each session | NR | Throughout the study | |||||
| Liabsuetrakul 2017 | Yes |
|
Audit and feedback | Medical practitioners (all general practitioners and nurses who were involved in the provision of labor and 24‐hour post‐delivery services) | Educational PPH seminar | Single session | Once | 2 months | Usual care with audit and feedback |
| Conduct educational outreach visits | |||||||||
| Inform local opinion leaders | NR | NR | NR | ||||||
| Remind clinicians | Posted PPH algorithm | Every labor room | Throughout the study | ||||||
| Sloan 2005 | No2 |
|
Change physical structure and equipment | Qualified health professionals (basic midwifery training with 1 year clinical practice), specialists (obstetricians) | Essential equipment | As needed | Throughout the study | 2 weeks | Usual care |
| Use train‐the‐trainer strategies | LSS training | Single session | Daily for 2 weeks | ||||||
| Awaiting classification | |||||||||
| Jayanna 2016 | No2 |
|
Change record systems | Nurses | Onsite training by RN | 2 days of training | Once | NR | Refresher training and case sheet added |
| Purposely re‐examine the implementation | |||||||||
| Create a learning collaborative | |||||||||
| Conduct educational outreach visits | NR | ||||||||
| Conduct ongoing training | Every 2 months for 3‐day site visit | ||||||||
| Abbreviations: AIP: Advances in Labour and Risk Management (ALARM) International Program (AIP); EBL: estimated blood loss; HMS BAB: Helping Mothers Survive Bleeding After Birth; LSS: Lifesaving Skills; MD: medical doctor; MCSSL: Modified World Health Organization surgical safety checklist; NR: not reported; OSCE: Objective Structured Clinical Examination; PPH: postpartum hemorrhage; RN: registered nurse | |||||||||
1Implementation strategy domain as outlined in Waltz and colleagues [9]. 2Studies that were not primarily focused on PPH were focused on the following topics: Althabe 2008: Obstetric care; Evans 2018: Helping Babies Breathe & Helping Mothers Survive training program; Klokkenga 2019: Safe Delivery App covered topics beyond the scope of PPH; Naidoo 2017: Surgical Checklist on topics related to surgical obstetric care; Mogilevkina 2022: Topics included obstructed labor, hemorrhage, sepsis, hypertensive disorders and complications due to unsafe abortion; Sloan 2005: Obstetric care; Jayanna 2016: Obstetric care.
Comparisons and control groups
Seven studies used usual care as the comparison (Al‐beity 2019; Hanson 2021; Klokkenga 2019; Mogilevkina 2022; Naidoo 2017; Sloan 2005; van de Ven 2017). Six studies provided an enhancement to usual care in the comparison group:
Althabe 2008 provided standard in‐service training for the comparison facilities [51];
Deneux‐Tharaux 2010 passively disseminated the early management of PPH protocol at staff meetings in control facilities;
Evans 2018 provided the same one‐day training in both the control and implementation facilities;
Gallos 2024 provided blood collection drapes without an alert or action line to the control facilities;
Main 2017 utilized modified data collection strategies in both comparison and implementation facilities;
Liabsuetrakul 2017 conducted the same audit and feedback cycles in comparison and implementation facilities.
Interventions tailored to WHO guidelines
A total of 39 potential WHO recommendations or evidence‐based practices were under consideration for the WHO PPH guideline review committee to which this review contributes. Of these, 14 were either new practices, which have yet to be defined, or were clinical practices outside the health facility or in research settings only, thus we included 25 WHO recommendations. Of these, 10 recommendations focused on prevention, 1 focused on PPH detection, and 14 addressed management of PPH.
The included studies addressed 1 to 14 WHO PPH guidelines (Table 2). The most commonly addressed guidelines focused on the detection of PPH with estimation of blood loss (10 studies: Al‐beity 2019; Althabe 2008; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Main 2017; Mogilevkina 2022; Naidoo 2017), prevention of PPH via uterotonic use (11 studies: Al‐beity 2019; Althabe 2008; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Liabsuetrakul 2017; Main 2017; Mogilevkina 2022; Sloan 2005) and treatment of PPH with uterine massage (9 studies: Al‐beity 2019; Althabe 2008; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Liabsuetrakul 2017; Mogilevkina 2022; Sloan 2005) and IV oxytocin (10 studies: Al‐beity 2019; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Liabsuetrakul 2017; Main 2017; Mogilevkina 2022; Sloan 2005). Of note, two prevention (late cord clamping; controlled cord traction without a skilled birth attendant) and three treatment recommendations (avoid uterine packing; use of non‐pneumatic anti‐shock garments; antibiotics for manual removal of placenta) from WHO were not addressed in any of the included studies (Table 2).
Implementation strategies
We used the ERIC classification to categorize the implementation strategies across the 13 included studies; see Table 5 [7, 9]. Five of nine higher‐level implementation domains were used: develop stakeholder interrelationships; train and educate stakeholders; change infrastructure; use evaluative and iterative strategies; and support clinicians. Of the 73 implementation strategies suggested by Powell and colleagues [7], 24 strategies were used in a variety of combinations. These 24 strategies included: audit and provide feedback; build a coalition; capture and share local knowledge; change physical structure and equipment; change record systems; conduct educational outreach visits; conduct local needs assessment; conduct ongoing training; create a learning collaborative; develop and organize quality monitoring systems; distribute educational materials; identify and prepare champions; inform local opinion leaders; mandate change; model and simulate change; promote network weaving; provide clinical supervision; provide local technical assistance; remind clinicians; reminders to clinicians; support clinicians; tailor strategies; use data experts; and use train‐the‐trainer strategies. The implementation approaches of each study and combinations of strategies used are described in Table 8 and Table 9.
6. Implementation strategy details, by study*.
| Study | Implementation strategy: details |
| Randomized studies | |
| Althabe 2008 | Three to six birth attendants trained in a five‐day workshop to develop evidence‐based guidelines on the management of the third stage of labor and the use of episiotomy. Then had a one‐day workshop to build training skills. These teams then disseminated the guidelines, trained and visited birth attendants, and developed reminders to be placed in labor and delivery wards, inside surgical packages for birth attendants, and on clinical records. Monthly reports on key indicators compiled by these trainers. Regional co‐ordinators met monthly with each team. Each intervention hospital received a computer with intervention materials installed on it, copies of the guidelines, the WHO Reproductive Health Library, and BMJ Clinical Evidence. |
| Al‐beity 2019 | HMS BAB program used a low‐fidelity simulator—the Mama Natalie; one‐day training on HMS BAB and six to eight weeks of weekly drills/practice (30 to 40 minutes duration) in the facility (Low Dose, High Frequency); HMS BAB master trainers, two trainers per district local HMS BAB trainers identified and conducted follow‐up; one per facility peer practice co‐ordinator. HMS BAB‐trained master trainers facilitated the training of 12 district trainers in a central training. Then six pairs of HMS BAB district trainers were accompanied by Jhpiego master trainers to intervention districts. The district trainers were paired, each pair conducted their first in‐facility training under the supervision of Jhpiego master trainers to ensure their adherence to training standards. Each pair spent 1.5 days per facility conducting the training. Day 1 was for HMS training for all providers; day 2 was spent coaching two local providers to become facility peer practice co‐ordinators. Providers who excelled in the skills training, were willing to lead others, and had good communication skills were selected to be peer practice co‐ordinators and received this half‐day training and coaching. Step 3 facilitate six to eight weekly drills and exercises using specific scenarios to augment the learned skills, led by facility peer practice co‐ordinator. |
| Deneux‐Tharaux 2010 | The components of the intervention were implemented in two phases. The first phase lasted three months and consisted of outreach visits to each maternity unit (two visits per unit). During these visits, the team presented the key points of the protocol to the local target clinicians (obstetricians, midwives, and anesthetists) and discussed with them possible difficulties in its local implementation as well as potential solutions. Each intervention unit received a color poster summarizing the key steps of the protocol. In addition, a specific ‘PPH chronological check list’ was provided, a simple graphic reminder of the recommended steps for PPH management with a time scale on one side to be filled in by the care provider for each woman with PPH. Writing down the time of delivery, of PPH diagnosis, and each procedure undertaken as the event occurred was intended to increase awareness of potential delays in care and to support decision‐making in these women. Finally, a ‘PPH box’ was given to each unit. This box was intended to provide a single place where all drugs and materials needed for PPH management were available, a list of useful phone numbers, forms for transfusion orders and laboratory examinations, and a chronometer to keep exact track of the time. The second phase of the intervention was a peer review of deliveries with severe PPH, organized in each intervention unit. All incidences of severe PPH identified during the first three months of data collection were reviewed during one meeting of the trial team with the local clinicians. The quality of care provided was critically analyzed, and feedback on their practices provided to the local staff. For each network, an obstetrician and a midwife identified as opinion leaders in their professional community were teamed to implement the intervention’s components in each maternity unit. |
| Evans 2018 | We provided simulation‐based training in management of PPH and neonatal resuscitation to all facilities in all study groups using the HMS BAB training module in June 2014 and the HMS HBB training module in September 2014. Training was delivered as two separate one‐day team trainings at all facilities, and all providers on the labor ward were invited to participate. All 24 trainers passed ModCAL for Training Skills, a computer‐based course that helps learners become more effective trainers. Trainers were subsequently trained in BAB and HBB and were mentored by a master trainer during their first facility‐based training of each module prior to implementation. BAB and HBB trainings were purposefully spaced three months apart to allow for consolidation of learning by both trainers and providers. At the end of each training, all providers were instructed to practice specific scenarios using simulators for 10 to 15 minutes every week for eight weeks, and then combined maternal and newborn scenarios for four weeks for a total of 20 weeks of practice. Simulators were left at each facility for skills practice. We compared the training intervention alone (control group) with the same intervention with an added Peer‐Assisted Learning (PAL) component as follows. The full intervention and partial intervention groups received the training and practice intervention as described above. In addition, district trainers selected Clinical Mentors from each facility and oriented them to a PAL role to support onsite practice after training. Clinical Mentors were practicing midwives who were tasked with organizing and leading brief, structured practice sessions onsite, once per week with fellow providers, in addition to performing their clinical duties. In the full intervention group, as an additional element, district trainers made telephone calls to remind Clinical Mentors to facilitate practice. |
| Gallos 2024 | The E‐MOTIVE intervention consisted of a calibrated drape for early detection of PPH and the WHO first‐response treatment bundle, which included uterine massage, oxytocic drugs, tranexamic acid, intravenous fluids, and a process for examination and escalation. Implementation was supported by several components, including the use of trolleys or carry cases for PPH; simulation‐based, onsite training; local champions (midwives and doctors who lead and support change in participating hospitals); and audit and feedback of actionable data to providers. The implementation strategy was informed by the findings from our formative research and refined during multidisciplinary workshops in each of the participating countries. The intervention was piloted and refined in three hospitals in each country that did not participate in the main trial. Restocking of all medicaine and devices used for treatment of PPH was done; audit newsletters that shared with all staff rates of detection and bundle use, along with rates of key outcomes. Feedback was also given at departmental meetings; feedback given with audit newsletters, connect with other champions by means of chats, meetings, and websites for sharing knowledge and lessons learned; calibrated drape for early detection of PPH; use of trolleys or carry cases for PPH; onsite, simulation‐based, and peer‐assisted training facilitated by the use of provider guides, flipcharts, and job aids displayed in labor wards. Content included uterine massage, oxytocic drugs, tranexamic acid, intravenous fluids, and a process for examination and escalation. |
| Hanson 2021 | HMS BAB program using a low‐fidelity simulator—the Mama Natalie. One‐day training on HMS BAB + six to eight weeks of weekly drills/practice (30 to 40 minutes duration) in the facility (Low Dose, High Frequency); prevention and basic care for PPH, reinforcing skills and knowledge taught during the primary midwifery training; HMS BAB master trainers, two per district local HMS BAB trainers identified and conducted follow‐up; one per facility peer practice co‐ordinator (245 providers trained in intervention arm). Peer practice co‐ordinators were reminded by phone calls for a period of three weeks to initiate the in‐facility drills. |
| Klokkenga 2019 | The Safe Delivery app is an Emergency Obstetric and Neonatal Care (EmONC) training tool that provides visual guidance by means of animated videos with clinical instructions. It was developed for birth attendants in low‐ and middle‐income countries and aims to improve the quality of maternal and neonatal care. The videos give vivid instructions of, for example, how to administer drugs and perform maneuvers. The version we tested contained videos of: (1) active management of the third stage of labor, (2) manual removal of the placenta, (3) treatment of PPH, and (4) neonatal resuscitation. Midwives watched the videos once while the principal investigator was present. Thus, all midwives watched the app at least once. |
| Naidoo 2017 | The intervention on the use of the modified WHO surgical safety checklist (MSSCL) consisted of training by the principal investigator (MN) of doctors and nurses working in maternity operating theaters during May 2013. The MSSCL used was the SSCL adapted by the provincial health department of the Western Cape Province of South Africa and further modified by the study team (deleted an item on scalp vein electrodes). The MSSCL consists of three sections: the sign‐in phase, the time‐out phase and the sign‐out phase. |
| van de Ven 2017 | Team training was delivered using high‐fidelity mannequins by a gynecologist and a communication expert. All training instructors underwent an instructor training course for running simulation‐based trainings, with an emphasis on crew resource management. Every scenario started with a briefing by an introductory video for approximately 5 minutes, where the clinical situation is performed by actors on a wide screen. After this introduction, the team moved to the simulation delivery room where they were required to manage the simulated patient. All the scenarios, which lasted approximately 15 minutes, were videotaped. After finishing each scenario, the team returned to the briefing room for a 30‐minute debriefing session. Feedback on teamwork and the application of medical technical skills was provided by reviewing the relevant video recordings. Feedback on teamwork concentrated on components of crew resource management (i.e. communication, leadership, decision‐making, and situational awareness). All individual teams from each hospital were trained within a time period of four weeks. More than six months after completing their training courses, an unannounced in situ clinical simulation with two scenarios, shoulder dystocia and amniotic fluid embolism, was conducted at all obstetric units in both the intervention and control groups recruited into the study. At the time of their training, participants were aware that an unannounced in situ simulation would take place more than six months later. |
| Non‐randomized studies | |
| Main 2017 | Two learning collaboratives with 25 to 30 volunteer California hospitals were undertaken in 2011 and 2013. Subsequent key informant interviews with participants were used to design this statewide implementation project. The collaborative content followed the organization of the National Partnership for Maternal Safety Consensus Bundle for Obstetric Hemorrhage with four domains (readiness, recognition and prevention, response, and reporting/systems improvement). Each of these domains has a series of recommended bundle elements. This was the mentor model, wherein a physician and nurse pair with maternal quality improvement experience were matched with groups of five to eight hospitals. The hospital groups were often geographic or system‐based. The mentors were not from the facilities they supported, but they served as facilitators leading the monthly telephone calls, providing small group leadership and personal accountability. A CMQCC staff member also supported the mentor groups and attended all telephone calls to co‐ordinate and share lessons and ideas from all the groups. In‐person, full‐day meetings for learning and sharing involving all hospital teams were held toward the beginning and the end of the project. Additionally, hospitals were encouraged to share resources and discussions on a collaborative electronic mail list/resource‐sharing service. A key feature of the collaborative was the use of the CMQCC Maternal Data Center for data collection of structure, process, and outcome measures. The maternal data center is a rapid‐cycle system that minimizes data collection burden, designed in partnership with state agencies. |
| Mogilevkina 2022 | The Advances in Labour and Risk Management (ALARM) International Program (known collectively as AIP) training educational environment includes interactive plenary sessions and hands‐on skill workshops with obstetrical mannequins, thus allowing all participants the opportunity to address individual learning needs. Sessions were accompanied by pre‐ and post‐tests which measured knowledge and practice skills, including objective structured clinical examinations that required the participant to demonstrate clinical skills. A two‐hour monitoring and refresh session on PPH was conducted in the case units in late 2009. |
| Liabsuetrakul 2017 | The multifaceted intervention program performed in the present study included educational outreach, a medical record audit, and the provision of feedback to medical practitioners based on that audit, reminders in the form of a PPH management chart posted in labor rooms and the postpartum ward, and the involvement of opinion leaders including the senior general practitioners and nurses of each hospital, who approved the audit and educational outreach and supported the implementation of the intervention. At the intervention hospitals, an audit of medical records of women diagnosed with PPH between 1 January 2011 and 31 October 2012 was performed by two researchers (TL and NO). Subsequently, the research team visited the intervention hospitals during January and February 2013 to deliver educational outreach in the form of one seminar session that emphasized the burden of PPH and the importance of proper PPH management; the session included feedback based on the results of the PPH management audit. The medical record audit was based on an essential PPH management guideline developed and published in a previous study. All participants in the intervention were asked to evaluate each PPH management item in terms of whether it was relevant, measurable, improvable, and acceptable using a self‐administered questionnaire that was collected at the end of the seminar. After the seminar, the PPH management chart was given to the senior general practitioners and nurses of each hospital to act as a reminder of the PPH management policies. It was explained to all seminar participants that the senior general practitioners and nurses of their hospital supported the intervention and the aim of improving PPH management. Medical records from women diagnosed with PPH between 1 March 2013 and 31 December 2014, after the intervention was completed, were reassessed by the same researchers to audit PPH management practices using the same guidelines used in the pre‐intervention audit. The audit process was completed on 30 April 2015. |
| Sloan 2005 | An LSS training center was created at Hung Vuong Hospital, a high‐volume hospital in Ho Chi Minh City in 1995. The American College of Nurse‐Midwives’ Lifesaving Skills manual was translated into Vietnamese. Expert LSS trainers from the American College of Nurse‐Midwives conducted a two‐week intensive, competency‐based training for practitioners from Hung Vuong Hospital to prepare them as master LSS trainers. These trainers then provided two weeks of LSS competency‐based training to qualified healthcare professionals from the intervention districts’ clinics and district hospitals. Qualified professionals had basic midwifery training and at least one year of clinical practice, specifically in delivery. Specialists (obstetricians) from the intervention districts’ hospitals received one‐week LSS and one‐week refresher training in performing cesarean sections. Essential equipment and supplies were provided to the intervention groups’ healthcare facilities as needed (determined by a pre‐intervention assessment of all study area public health facilities). Vacuum extractors were given to hospitals from which obstetricians were trained. |
| Studies awaiting classification | |
| Jayanna 2016 | The onsite mentoring intervention focused on improving systems and staff functioning within each facility. The 54 intervention facilities received six supportive onsite visits by one of nine nurse mentors during the course of one year (a dedicated nurse mentor for six facilities). The mentors had a basic qualification in general nurse midwifery (GNM), and were recruited locally. They were given further training for five weeks on topics related to quality improvement approaches and tools, mentoring skills, and clinical topics on obstetric and neonatal care and primary care systems. In addition, they received handholding support in the field once every quarter by clinical experts who helped in reviewing and reinforcing the mentoring skills of the mentors. The mentors attended clinical refresher every six months to renew their clinical skills and practices. The trained mentors visited facilities allocated to them once every two months, with each visit lasting for three days. During the visits, they trained the staff in using self‐assessment checklists to assess gaps in facility readiness, and develop action plans to address gaps that were identified. Clinical mentorship was provided using multiple approaches such as bedside coaching, case demonstrations, use of case vignettes, and job‐aids. The mentors particularly focused on care during intrapartum and postpartum periods including essential newborn care alongside recognition, management, and referral of common complications during these periods. They used a structured teaching plan during each visit, yet were flexible in responding to the emerging needs in the facilities. Case sheet audits and observations of staff practices helped mentors to plan the clinical mentoring sessions with the staff either in one‐to‐one or one‐to‐group sessions as appropriate. Each visit started with a planning meeting and ended with a debrief meeting with the facility teams that ensured joint planning, follow‐up, and continuity. Prior to the mentoring program, a new case sheet was introduced to the staff nurses and medical officers at all 108 facilities (both intervention and control) through a training update of three days and one day duration, respectively. The new case sheets were developed by the project in consultation with the Karnataka government and piloted as a part of the mentoring intervention. The earlier case sheets were open‐ended and not specific to maternal and newborn care. They were restructured to function as job‐aid in providing step‐by‐step guidance to staff for managing women during initial assessment, labor monitoring, delivery, and postpartum care, and to facilitate diagnosis of maternal and neonatal complications and their pre‐referral management. The restructured case sheets consisted of a delivery record and eight separate complication sheets, one for each of the most common complications (maternal: prolonged/obstructed labor, preeclampsia and eclampsia, antepartum hemorrhage, infection/sepsis, premature rupture of membranes, PPH; newborn: neonatal asphyxia, sepsis, low birthweight/prematurity). Orientation training was provided to refresh the providers’ knowledge and skills. |
CMQCC: California Maternal Quality Care Collaborative; HBB: Helping Babies Breathe; HMS BAB: Helping Mothers Survive Bleeding after Birth; LSS: Lifesaving Skills; PPH: postpartum hemorrhage; WHO: World Health Organization
*Implementation strategy detail abstracted directly from study manuscript or study protocol, or both, with minor editorial changes to improve readability.
Excluded studies
We excluded 22 studies at the full‐text stage for the following reasons:
no comparator group (8 studies);
abstract only (6 studies);
no clinical outcomes (5 studies);
no WHO recommendation (2 studies);
not a clinical study (1 study).
Further details are provided in Supplementary material 3.
Studies awaiting classification
After applying the Cochrane Pregnancy and Childbirth Trustworthiness Screening Tool to all included studies, we contacted study authors/investigators to request additional information [29]. For the one study awaiting classification (Jayanna 2016), we have not received appropriate information to confirm the study's trustworthiness (as shown in Figure 3) or details to determine categorization. See Supplementary material 4.
Ongoing studies
There are three ongoing studies (ISRCTN17679951 2023; jRCT1090220328 2018; Kamala 2021). Further details are provided in Supplementary material 5.
Risk of bias in included studies
Overall risk of bias: cluster‐RCTs
We assessed methodological and risk of bias for nine cluster‐RCTs, which contributed results to all critical outcomes using the adapted RoB 2 tool for cluster‐RCTs. The trials contributed results to the following eight outcomes.
Adherence to WHO prevention recommendation
Adherence to WHO treatment recommendation
PPH ≥ 500 mL within 24 hours after birth
PPH ≥ 1000 mL within 24 hours after birth
Blood transfusion during hospitalization for birth
Maternal death up until discharge for birth hospitalization and up to 42 days after birth
Severe morbidity (outcomes related to ICU admission)
Severe morbidity (surgical outcomes)
The risk of bias judgments are summarized below and presented in Figure 1. Detailed consensus risk of bias assessments are provided in Supplementary material 7, and the full consensus responses to the entire RoB 2 tool are available upon request.
Overall risk of bias by outcome
Adherence to WHO prevention recommendation
Three trials reported on this outcome (Althabe 2008; Evans 2018; Klokkenga 2019). We assessed two trials as at high risk of bias: Klokkenga 2019 failed to report allocation concealment, recruited participants after clusters were randomized, and did not provide an a priori analysis plan, while Evans 2018 did not report methods for several domains. We assessed Althabe 2008 as some concerns due to insufficient detail describing the method of allocation concealment.
Adherence to WHO treatment recommendation
Four trials reported on this outcome (Al‐beity 2019; Deneux‐Tharaux 2010; Gallos 2024; Hanson 2021). We assessed Gallos 2024 as at low risk of bias for this outcome. We judged two studies to be at high risk of bias due to the measurement of adherence (Deneux‐Tharaux 2010; Hanson 2021). We judged Al‐beity 2019 to be some concerns due to lack of an a priori analysis plan.
PPH ≥ 500 mL
Eight trials reported on this outcome (Al‐beity 2019; Althabe 2008; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024; Hanson 2021; Klokkenga 2019; Naidoo 2017). We judged Gallos 2024 to be at low risk of bias for this outcome. We judged four studies to be at high risk of bias, the most common reason being the measurement of PPH (Deneux‐Tharaux 2010; Evans 2018; Hanson 2021), while Klokkenga 2019 did not report the method of allocation concealment, recruited participants after clusters were randomized, and did not provide an a priori analysis plan. We assessed the remaining three trials as some concerns (Al‐beity 2019; Althabe 2008; Naidoo 2017). Baseline imbalances suggested a problem with randomization in Althabe 2008 and Naidoo 2017. Additionally, Althabe 2008 did not report the method of allocation concealment, while Naidoo 2017 had some concerns for measurement of PPH. Al‐beity 2019 did not specify an a priori analysis plan.
PPH ≥ 1000 mL
Four trials reported on this outcome (Althabe 2008; Deneux‐Tharaux 2010; Gallos 2024; Klokkenga 2019). We assessed Gallos 2024 as at low risk of bias for this outcome. We judged two studies to be at high risk or bias: Deneux‐Tharaux 2010 due to the measurement of PPH, and Klokkenga 2019 due to lack of reporting of method of allocation concealment, recruiting participants after clusters were randomized, and not providing an a priori analysis plan. We judged Althabe 2008 to be some concerns due to baseline imbalance in PPH rate between groups and insufficient reporting of the method of allocation concealment.
Blood transfusion
Five trials reported on this outcome (Al‐beity 2019; Deneux‐Tharaux 2010; Gallos 2024; Hanson 2021; Klokkenga 2019). We assessed Gallos 2024 as at low risk of bias for this outcome. We judged two trials to be some concerns for reporting bias, as neither study provided an a priori analysis plan (Al‐beity 2019; Deneux‐Tharaux 2010). There were additional concerns about the measurement of PPH in Deneux‐Tharaux 2010. We assessed the remaining two trials as at high risk of bias (Hanson 2021; Klokkenga 2019). Hanson 2021 did not blind outcome assessors or report methods of outcome assessment, and Klokkenga 2019 did not report the method of allocation concealment, recruited participants after clusters were randomized, and did not provide an a priori analysis plan.
Maternal death
Seven trials reported on this outcome (Al‐beity 2019; Althabe 2008; Gallos 2024; Hanson 2021; Klokkenga 2019; Naidoo 2017; van de Ven 2017). We judged Gallos 2024 as at low risk of bias for this outcome. Four trials had some concerns (Al‐beity 2019; Althabe 2008; Hanson 2021; Naidoo 2017). In Althabe 2008 and Naidoo 2017 baseline imbalances suggested a problem with randomization; Hanson 2021 did not provide information to permit a judgment on deviations from intended interventions; and Al‐beity 2019 did not specify an a priori analysis plan. We assessed two trials as at high risk of bias (Klokkenga 2019; van de Ven 2017). Klokkenga 2019 did not report the method of allocation concealment, recruited participants after clusters were randomized, and did not provide an a priori analysis plan. van de Ven 2017 provided insufficient information on allocation concealment, blinding, missing outcome data, and a priori analysis plan.
Severe morbidity (outcomes related to ICU admission)
Three trials reported on this outcome (Althabe 2008; Gallos 2024; Naidoo 2017). We judged Gallos 2024 to be at low risk of bias for this outcome. Althabe 2008 and Naidoo 2017 had some concerns about baseline imbalances in the treatment groups. Additionally, Althabe 2008 did not report the method of allocation concealment, while Naidoo 2017 had some concerns about the measurement of the outcome.
Severe morbidity (surgical outcomes)
Four trials reported on this outcome (Al‐beity 2019; Gallos 2024; Hanson 2021; Naidoo 2017). We judged Gallos 2024 to be at low risk of bias for this outcome. Three trials had some concerns (Al‐beity 2019; Althabe 2008; Naidoo 2017). Baseline imbalances between the intervention and control groups raised concerns about the randomization process in Al‐beity 2019 and Naidoo 2017. Other reasons for concern were lack of allocation concealment in Althabe 2008, bias in the measurement of the outcome in Naidoo 2017, and no a priori analysis plan in Al‐beity 2019. We judged Hanson 2021 as at high risk of bias, as the methods for outcome assessment were unclear, and outcome assessors were not blinded. Morbidity is a composite outcome made up of many subjective measures, hence the judgment of 'high risk of bias' rather than 'some concerns.'
Overall risk of bias: NRSIs
We assessed methodological and risk of bias for four NRSIs contributing results to all critical outcomes using the ROBINS‐I tool for NRSIs. The studies contributed results to the following five outcomes.
PPH ≥ 500 mL within 24 hours after birth
PPH ≥ 1000 mL within 24 hours after birth
Blood transfusion during hospitalization for birth
Maternal death up until discharge for birth hospitalization and up to 42 days after birth
Severe morbidity (surgical outcomes)
The risk of bias judgments are summarized below and presented in Figure 2. Detailed consensus risk of bias assessments are provided in Supplementary material 8; the full consensus responses to the entire ROBINS‐I tool are available upon request.
Overall risk of bias by outcome
Adherence to WHO treatment recommendation
Liabsuetrakul 2017 reported on this outcome and was judged to be at serious risk of bias due to important confounding domains not being controlled for.
PPH ≥ 500 mL
Two studies reported on this outcome, both of which were judged to be at serious risk of bias due to important confounding domains not being controlled for and because the analysis did not account for major differences in the start of follow‐up and start of intervention for most participants (Main 2017; Sloan 2005). Sloan 2005 was also at serious risk of bias due to deviations from the intended interventions and in the measurement of PPH.
PPH ≥ 1000 mL
Two studies reported on this outcome, both of which were judged to be at serious risk of bias due to important confounding domains not being controlled for and because the measurement for PPH differed between intervention and control cohorts (Liabsuetrakul 2017; Mogilevkina 2022).
Blood transfusion
Two studies reported on this outcome, both of which were judged to be at serious risk of bias due to important confounding domains not being controlled for (Liabsuetrakul 2017; Mogilevkina 2022), and because the measurement for blood transfusions differed between intervention and control cohorts in Mogilevkina 2022.
Maternal death
Two studies reported on this outcome, both of which were judged to be at serious risk of bias due to important confounding domains not being controlled for (Mogilevkina 2022; Sloan 2005). Sloan 2005 was also at serious risk of bias for two other domains, selection of participants and deviations from intended interventions.
Severe morbidity (surgical outcomes)
Main 2017 was the only study to report on this outcome and was judged to be at serious risk of bias due to important confounding domains not being controlled for and because the analysis did not account for major differences in the start of follow‐up and start of intervention for most participants.
Synthesis of results
Single‐component implementation strategies versus usual care
Three studies with 86,788 births met the criteria for comparing a single implementation strategy versus usual care (Klokkenga 2019; Naidoo 2017; van de Ven 2017). See Table 1.
Adherence to WHO PPH prevention recommendations
One study with 147 midwives as participants used a single‐component 'train and educate' implementation strategy, which consisted of a Safe Delivery App that contained instructional videos about emergency obstetric and neonatal care (Klokkenga 2019). The study authors reported improved adherence to reducing the routine use of episiotomy (1% implementation arm, 10% in control), and no difference in adherence to the use of oxytocin for active management of the third stage of labor (AMTSL) (97% in the implementation arm, 96% in the control). Naidoo 2017 and van de Ven 2017 did not report adherence to guidelines in a way that could be interpreted. The evidence is of very low certainty, thus we do not know if single‐component implementation strategies have any effect on adherence to WHO PPH prevention recommendations.
PPH ≥ 500 mL within 24 hours after birth
Two cluster‐RCTs involving 30,396 participants measured this outcome, but the certainty of the evidence is very low. Klokkenga 2019 reported a reduction in PPH ≥ 500 mL (odds ratio [OR] 0.86, 95% confidence interval [CI] 0.59 to 1.25). Naidoo 2017 found an incidence rate ratio (IRR) of 0.91 (95% CI 0.66 to 1.28; P = 0.61). We were unable to pool data due to heterogeneity of implementation strategies, outcomes measures, and time points. We do not know if single‐component implementation strategies have any effect on PPH ≥ 500 mL.
PPH ≥ 1000 mL within 24 hours after birth
Klokkenga 2019 reported a reduction in PPH ≥ 1000 mL (OR 0.95, 95% CI 0.65 to 1.40; 3411 births). The evidence is of very low certainty, thus we do not know if single‐component implementation strategies have any effect on PPH ≥ 1000 mL.
Blood transfusion during hospitalization for birth
One cluster‐RCT involving 3411 participants found no difference in blood transfusions between groups; they reported raw numbers with no statistical testing (Klokkenga 2019). The evidence is of very low certainty, thus we do not know if single‐component implementation strategies have any effect on blood transfusion.
Maternal death up until discharge for birth hospitalization and up to 42 days after birth
Three cluster‐RCTs involving 86,788 births reported no difference in maternal mortality, based on low‐certainty evidence.
Klokkenga 2019: implementation arm: 3 deaths/1665 women; control: 3 deaths/1746 women.
Naidoo 2017: implementation arm: pre: 14 deaths/5418 women, post: 15 deaths/10,479 women; control: pre: 4 deaths/3731 women, post: 8 deaths/7357 women.
van de Ven 2017: implementation arm: pre: 0 deaths/13,971 women, post: 0 deaths/14,500 women; control: pre: 1 death/13,538 women, post: 1 death/14,517 women.
Overall, single‐component implementation strategies may have little or no impact on maternal death.
Severe morbidity (outcomes related to ICU admission)
Naidoo 2017 reported an increase in referral to a higher level of care, including ICU admission (IRR 1.41, 95% CI 1.07 to 1.86; 26,985 births), based on low‐certainty evidence.
Severe morbidity (surgical outcomes)
Naidoo 2017 reported a decrease in unscheduled return to the operating theater (IRR 0.72, 95% CI 0.57 to 0.90; 26,985 births), based on low‐certainty evidence.
Other outcomes
The following outcomes were not measured in studies comparing single‐component implementation strategies versus usual care: adherence to WHO PPH treatment recommendations, additional uterotonics within 24 hours after birth, adverse effects (variable and related to the implementation strategy) during hospitalization for birth, and our predefined important outcomes (see Important outcomes).
Multicomponent implementation strategies versus usual care
Four studies with 466,485 births met the criteria for comparing a multicomponent implementation strategy versus usual care (Al‐beity 2019; Hanson 2021; Mogilevkina 2022; Sloan 2005). See Table 3.
Adherence to WHO PPH treatment recommendations
Two cluster‐RCTs with the same protocol [52] found opposite results in adherence to uterotonics for treatment of PPH. Al‐beity 2019, conducted in Tanzania, reported improved adherence (difference in differences [DiD] in slopes 5.2, 95% CI 1.4 to 8.9), while Hanson 2021, conducted in Uganda, reported less adherence to WHO recommendation for treatment after the implementation strategy (DiD in slopes −4.02, 95% CI −6.74 to −1.30). The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on adherence to WHO PPH treatment recommendations.
Two NRSIs, Mogilevkina 2022 and Sloan 2005, did not report adherence in a way that could be interpreted.
PPH ≥ 500 mL within 24 hours after birth
Two cluster‐RCTs involving 274,008 participants reported different effects on PPH ≥ 500 mL: Hanson 2021 found an increase in PPH measured by the long‐term difference effect of DiD in slopes 0.53 (95% CI 0.15 to 0.92), while Al‐beity 2019 reported a decrease in PPH ≥ 500 mL (DiD in slopes −0.3, 95% CI −0.7 to 0.1). The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on PPH ≥ 500 mL within 24 hours after birth.
One NRSI, Sloan 2005, reported no difference in PPH ≥ 500 mL across the three study groups (0.4% control; 0.3% hospital only; 0.2% hospital and clinic).
Blood transfusion during hospitalization for birth
Two cluster‐RCTs involving 274,008 participants reported on blood transfusion during hospitalization for birth: Hanson 2021 reported no difference in differences in slopes in the long term (−0.35, 95% CI −2.59 to 1.88), whereas Al‐beity 2019 reported a DiD in slopes of −8.0 (95% CI −12.6 to −3.4; P < 0.01). The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on blood transfusion during hospitalization for birth.
Mogilevkina 2022, an NRSI, found a reduction in blood transfusions in implementation facilities (OR 0.56, 95% CI 0.48 to 0.65).
Maternal death up until discharge for birth hospitalization and up to 42 days after birth
Two cluster‐RCTs involving 274,008 participants measured maternal mortality, both of which found no difference in maternal deaths, based on low‐certainty evidence.
Hanson 2021 reported the PPH case fatality rate: implementation arm: pre: 34 deaths/30,443 women, post: 53 deaths/50,992 women; control: pre: 64 deaths/27,557 women, post: 89 deaths/44,463 women.
Al‐beity 2019: implementation arm: pre: 44 deaths/24,437 women, post: 57 deaths/42,033 women; control: pre: 40 deaths/19,790, post: 61 deaths/34,383 women.
Two NRSIs, Mogilevkina 2022 and Sloan 2005, found no change in maternal mortality. Mogilevkina 2022 reported an OR of 1.64 (95% CI 0.35 to 7.66; P = 0.490), while Sloan 2005 reported one death in the control arm and one death in the hospital and clinic arm, but no deaths in the hospital arm.
Multicomponent implementation strategies may have little or no impact on maternal death.
Severe morbidity (surgical outcomes)
Two cluster‐RCTs involving 274,008 participants measured this outcome (Al‐beity 2019; Hanson 2021). Both trials specifically measured changes in PPH near‐misses among women who experienced PPH during their health facility delivery, and both trials found a reduction. The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on severe morbidity (surgical outcomes).
Hanson 2021 reported a reduction in PPH near‐misses in the implementation versus control arms: implementation arm: pre: 512 cases/30,443 births; post: 784/50,992; control: pre: 965/27,557; post: 1183/44,463; ITS −4.19, 95% CI –7.64 to –0.74, as did Al‐beity 2019: implementation arm: pre: 383 cases/24,347 births, post: 604/42,033; control: pre: 278/19,790, post: 559/34,282; ITS −5.3, 95% CI −7.8 to −2.7.
Other critical outcomes
The following critical outcomes (see Critical outcomes) were not measured in studies comparing multicomponent implementation strategies versus usual care: adherence to WHO PPH prevention recommendations, PPH ≥ 1000 mL within 24 hours after birth, additional uterotonics within 24 hours after birth, severe morbidity (outcomes related to ICU admission), and adverse effects (variable and related to the implementation strategy) during hospitalization for birth.
Important outcomes
Among the 13 included studies, none reported on breastfeeding uptake, implementation factors, or implementation outcomes. Two cluster‐RCTs reported improved healthcare professional knowledge from baseline (Al‐beity 2019; Hanson 2021). No other studies reported healthcare professional knowledge.
Multicomponent implementation strategies versus enhanced usual care
Six studies with 472,922 births met the criteria for comparing multicomponent implementation strategies versus enhanced usual care (Althabe 2008; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024; Liabsuetrakul 2017; Main 2017). See Table 4.
Adherence to WHO PPH prevention recommendations
Two cluster‐RCTs involving 14,718 births reported an increase in adherence to the use of prophylactic uterotonics (Althabe 2008; Evans 2018):
Althabe 2008: from 2.1% pre to 83.6% post (implementation arm); from 2.6% pre to 12.3% post (control arm); difference in rate change 67.5% (95% CI 38.9 to 87.1);
Evans 2018: from 8% pre to 50% post (implementation arm); from 11% pre to 51% post (control arm).
Both trials used train educate, develop interest‐holder relationships, evaluate and iterative strategies. Evans 2018 had an additional component intended to support clinicians. Furthermore, Althabe 2008 reported a reduction in episiotomy use (absolute median difference −10.9, 95% CI −16.1 to −5.8). Multicomponent implementation strategies may improve adherence to WHO PPH prevention recommendations compared to enhanced usual care, based on low‐certainty evidence.
Main 2017, an NRSI, did not separate prevention from treatment.
Adherence to WHO PPH treatment recommendations
Two cluster‐RCTs involving 356,913 births compared a multicomponent implementation strategy versus enhanced usual care. Gallos 2024, conducted in Kenya, Nigeria, South Africa, and Tanzania, reported improved adherence to a treatment bundle (risk ratio [RR] 4.94, 95% CI 3.88 to 6.28). Deneux‐Tharaux 2010, conducted in France, reported mixed findings, with a decrease in adherence in PPH treatment with oxytocin (T4 recommendation) (OR 0.92, 95% CI 0.63 to 1.33) and an increase in adherence to sulprostone (T5 recommendation) (OR 1.45, 95% CI 0.99 to 2.13). These results are based on low‐certainty evidence.
One cluster‐RCT and one NRSI reported on uterine massage (Gallos 2024; Liabsuetrakul 2017). Gallos 2024 reported increased use of uterine massage for treatment of PPH in the implementation arm (RR 1.91, 95% CI 1.66 to 2.20). Liabsuetrakul 2017, an NRSI, reported no difference in uterine massage or oxytocin administration, but both groups had 100% use among PPH patients.
Main 2017, an NRSI, did not separate prevention from treatment.
Multicomponent implementation strategies may improve adherence to WHO PPH treatment recommendations compared to enhanced usual care.
PPH ≥ 500 mL within 24 hours after birth
Four cluster‐RCTs (Althabe 2008; Deneux‐Tharaux 2010; Evans 2018; Gallos 2024) and one NRSI (Main 2017) involving 452,999 participants assessed this outcome. Three trials found a decrease in PPH ≥ 500 mL within 24 hours of birth: Gallos 2024 (RR 0.51, 95% CI 0.44 to 0.60); Althabe 2008 (RR 0.55, 95% CI 0.29 to 0.91); and Evans 2018 (17% reduction). Deneux‐Tharaux 2010 found no difference in PPH ≥ 500 mL. The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on PPH ≥ 500 mL within 24 hours after birth when compared with enhanced usual care.
Main 2017, an NRSI, found an increase in PPH ≥ 500 mL: implementation arm: pre: 5.9%, post: 6.7% (13.3% increase); control: pre: 4.2, post: 4.3 (4.5% increase). Of note, volumetric measurement of PPH was not used in this study.
PPH ≥ 1000 mL within 24 hours after birth
Three cluster‐RCTs involving 371,631 participants reported this outcome. Two trials found a reduction in PPH ≥ 1000 mL:
Gallos 2024: RR 0.39 (95% CI 0.31 to 0.49);
Althabe 2008: ratio of median relative risk was 0.30 (95% CI 0.22 to 0.84).
Deneux‐Tharaux 2010 found no difference in PPH ≥ 1000 mL (OR 1.02, 95% CI 0.83 to 1.24).
The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on PPH ≥ 1000 mL within 24 hours after birth when compared with enhanced usual care.
Liabsuetrakul 2017, an NRSI, found no effect on PPH ≥ 1000 mL (15% and 17% in the implementation and control arms, respectively, P = 0.996).
Blood transfusion during hospitalization for birth
Two cluster‐RCTs involving 356,913 participants assessed this outcome. Gallos 2024 found an overall reduction in blood transfusions (RR 0.71, 95% CI 0.55 to 0.90), while Deneux‐Tharaux 2010 reported no difference (OR 1.13, 95% CI 0.88 to 1.44). The evidence is of very low certainty, thus we do not know if multicomponent implementation strategies have any effect on blood transfusion during hospitalization for birth when compared with enhanced usual care.
Liabsuetrakul 2017, an NRSI, found a lower rate of patients requiring blood transfusions in hospital in the implementation group (26%) compared to the control group (46%) (P = 0.011).
Maternal death up until discharge for birth hospitalization and up to 42 days after birth
Two cluster‐RCTs involving 224,850 births found no difference in maternal death, based on moderate‐certainty evidence. Althabe 2008 found no difference in mortality rates, as there was one event in each trial arm (implementation arm: pre: 1/2963 births, post: 1/2587; control: pre: 1/2503, post: 1/2366); Gallos 2024 also found no difference, as demonstrated by the absolute risk difference (implementation arm: 17 deaths/49,101 births; control 28/50,558; OR 0.73, 95% CI 0.40 to 1.31).
Multicomponent implementation strategies probably have little or no effect on maternal death compared to enhanced usual care.
Severe morbidity (outcomes related to ICU admission)
Two cluster‐RCTs involving 224,850 participants reported this outcome. Gallos 2024 found no difference in ICU admission, as demonstrated by the absolute risk difference (implementation arm: 7 admissions/49,101 births; control: 32/50,558; RR 0.70, 95% CI 0.12 to 4.05); Althabe 2008 also found no difference in ICU admission between arms (implementation arm: pre: 3 admissions/2963 births, post: 3/2587; control: pre: 1/2503, post: 3/2366). These results are based on moderate‐certainty evidence.
Multicomponent implementation strategies probably have no effect on severe morbidity (ICU admission) compared to enhanced usual care.
Severe morbidity (surgical outcomes)
One cluster‐RCT (210,132 participants) and one NRSI (81,368 participants) measured this outcome. Gallos 2024 reported little or no difference in severe morbidity related to surgical outcomes, specifically to laparotomy. While the risk ratio shows a difference, albeit very wide confidence intervals (RR 1.72, 95% CI 0.57 to 5.16), the absolute risk ranges from 7 in 50,558 (0.01%) in the control arm versus 12 in 49,101 (0.02%) in the implementation arm. This finding is based on moderate‐certainty evidence.
Main 2017, an NRSI, found a decrease in severe morbidity among women diagnosed with PPH; severe morbidity was measured by the occurrence of blood transfusion, cardio monitoring, conversion of cardiac rhythm, hysterectomy, operations on heart of pericardium, temporary tracheostomy and/or ventilation (implementation: pre: 22.7%, post: 18.0% [20.8% decrease]; control: pre: 28.6%, post: 28.2 [1.2% decrease]).
Other outcomes
The following outcomes were not measured in studies comparing multicomponent implementation strategies versus enhanced usual care: adverse events during hospitalization for birth and our predefined important outcomes (see Important outcomes).
Equity assessment
While studies were conducted in various countries, allowing for diversity in populations, we were not able to conduct a formal analysis to evaluate differences.
Reporting biases
We were not able to assess reporting bias given that we did not perform a meta‐analysis.
Discussion
This review was a novel collaboration between Cochrane and the WHO that aimed to evaluate the effectiveness of implementation strategies for WHO guidelines to prevent, detect, and treat PPH. Notably, we found no pragmatic, hybrid effectiveness‐implementation studies evaluating comprehensive implementation and clinical outcomes during and after the study. Rather, there were few implementation outcomes collected; no studies used pragmatic or adaptive designs; and the included cluster‐RCTs were conducted in relatively controlled environments that did not represent real‐world circumstances. This review is an important step forward in evaluating the evidence base for implementation strategies around WHO PPH guidelines.
Summary of main results
The 13 studies that met our inclusion criteria were conducted in a wide range of settings, the majority being resource‐limited settings. We categorized studies into three groups based on the implementation strategies used: single implementation strategy versus usual care, multicomponent implementation strategies versus usual care, and multicomponent implementation strategies versus enhanced usual care. There is moderate‐certainty evidence that multicomponent implementation strategies for PPH probably have little to no effect on severe morbidity (i.e. ICU admission or surgical outcomes) or death compared with enhanced usual care. There is low‐certainty evidence that multicomponent implementation strategies may improve adherence to WHO PPH prevention and treatment recommendations compared with enhanced usual care. We are very uncertain if single‐ or multicomponent implementation strategies have any effect on PPH ≥ 500 mL or 1000 mL or on blood transfusion.
We applied gold‐standard assessment tools, including RoB 2, ROBINS‐I, and GRADE, to assess the certainty of evidence. The most common reason for downgrading the certainty of evidence was the lack of external observers for ascertaining outcome measurement, which is expensive and rare in many settings. It is challenging to intervene in routine clinical care. Klokkenga found that after some time working on site, research assistants would fill in data even when midwives did not complete certain tasks [53]. They also found that research assistants were more likely to intervene clinically if they were midwives. It remains unknown whether any of the multicomponent interventions work under typical conditions outside highly resourced, controlled randomized trial settings.
We found that implementation strategies may increase adherence to WHO guidelines in one setting, not make any difference in another, and may even reduce adherence in other settings. In separate reports of the same trial protocol conducted in two different countries, Tanzania (Al‐beity 2019) and Uganda (Hanson 2021), the implementation strategy increased adherence to WHO treatment guidelines in one setting and reduced it in the other. Implementation outcomes such as acceptability, adoption, appropriateness, cost, feasibility, fidelity, penetration, and sustainability [20] were not reported by either study and would have been useful in further understanding these differences in effects.
Across the included randomized studies, Forbes and colleagues [54], Althabe and colleagues [51], Williams and colleagues [55], and Klokkenga [53] provided additional insights regarding barriers and facilitators of implementation strategies. Barriers included staffing, workload, inadequate time to perform additional tasks, lack of supplies and refrigeration, policies regulating the scope of practice such as prescribing and administering medications, perceived value around education, internal motivation, and perception of clinicians' adequate compliance and competency. For example, Forbes and colleagues reported that some study sites had a policy that midwives could not prescribe or administer tranexamic acid, which they changed so that the implementation and control groups would be more similar. Recommended facilitators across studies included phone support, organizational expectations of training, provision of financial incentives and/or compensation for roles, and policy changes. These findings imply that contextual factors, often unmeasured or not incorporated into analyses, likely play a key role in the relationship between implementation strategies and critical outcomes.
Cost‐effectiveness analyses performed by van de Ven and colleagues, Williams and colleagues, and Wiesehan and colleagues reflected the beneficial cost‐effectiveness of their respective interventions, but varied in input and outcome measures [56, 57, 58].
Limitations of the evidence included in the review
There was large variation in detail about interventions and implementation strategies (e.g. content, depth, frequency, and duration), precluding precise comparisons or formal analyses across studies. Most studies reported in such a way that it was unclear what proportion of providers participated fully in the implementation strategy (adoption) and/or what proportion of all births were reported in the study. There was also variation regarding included populations based on facility‐level staffing, volume of births, and mode of births. While studies adjusted for clustering at the facility level, there was no stratification of adherence to the intervention by facility, which may have provided insights into facility‐level factors that influenced adherence and outcomes. None of the included studies measured long‐term sustainability of the implementation strategies. There were multiple external factors (e.g. international attention, local advocacy, quality improvement efforts, workforce changes) that made it difficult to measure the true impact of interventions on outcomes. Not all studies addressed resource constraints by filling supply chain gaps of necessary medications and supplies.
Only a subset of WHO PPH guidelines were addressed in the included studies, and the majority focused on PPH prevention and early treatment rather than later and more invasive treatment. Five of the WHO PPH guidelines had no explicit interventions (two prevention guidelines: late cord clamping and controlled cord traction without a skilled birth attendant; three treatment guidelines: avoid uterine packing, antibiotic provision in case of manual removal of the placenta, and non‐pneumatic anti‐shock garments in treatment) (see Table 2). Five studies included quantitative measurement of blood loss (see Table 6), which is critical for precise diagnosis and treatment rather than relying on subjective estimation of blood loss. No studies addressed the surgical techniques or other procedure recommendations for treating PPH. These are likely more proximal to the more severe clinical outcomes and need to be addressed if we expect to see a reduction in severe morbidity or mortality. No study captured the full care pathway for PPH according to WHO guidelines. No study assessed patient experience, postpartum depression, or breastfeeding rates.
Only 24 out of the 73 ERIC implementation strategies were used across the included studies. Similarly, only five of nine ERIC implementation domains were covered: train and educate stakeholders, develop stakeholder interrelationships, change infrastructure, use evaluative and iterative strategies, and support clinicians. Many implementation strategies were not studied, such as data management, financial strategies, and engaging consumers. Only two studies addressed team communication, one in the operating room (Naidoo 2017) and one on the labor ward (van de Ven 2017).
Many studies lacked a comprehensive framework that linked implementation efforts, adherence to WHO recommendations, and patient outcomes; it is doubtful that a multicomponent intervention could address all factors that contribute to PPH‐related morbidity and mortality. There were no pragmatic hybrid study designs, which prevented a clear understanding of implementation processes and outcomes across studies. Without conducting a rigorous qualitative assessment of the implementation strategy's incorporation of local contextual factors, we were unable to determine if the implementation strategy was fit for the specific context in which it was tested. Seven of the 13 studies measured adherence to one or more of the WHO guidelines (Table 2). The application of determinant frameworks would allow a better understanding of implementation processes. More comprehensive interventions and implementation strategies need to be tested for both implementation outcomes and maternal outcomes.
The certainty of the evidence was predominantly low to very low throughout, regardless of the comparison or outcome. The most common reasons for downgrading were methodological limitations of the included studies and serious imprecision due to wide 95% CIs. For the comparison single‐component implementation strategies versus usual care, only one study reported on adherence to WHO PPH prevention recommendation, PPH ≥ 1000 mL, and blood transfusion. When only one study contributed evidence to a given outcome, we downgraded the certainty of evidence by one level for indirectness. For the comparison multicomponent implementation strategies versus enhanced usual care, we assessed the certainty of evidence for severe morbidity (outcomes related to ICU admission) as moderate.
Limitations of the review processes
Given the timeline allocated for this review, we faced time constraints that created trade‐offs in comprehensiveness. We had to make the decision to exclude studies that did not have a comparison group, despite the value these studies may have provided regarding successful implementation strategies that address local facilitators and barriers in a specific setting. We contacted study authors about data questions, but had a limited timeframe to allow for responses. We heard from some authors but not all. Our search query identified three ongoing studies that met inclusion criteria at the time of this review.
The multiple dimensions of this review hindered our ability to fully evaluate the literature from all angles. Because we decided to include eight clinical outcomes (two of which were not reported in any study), we could only include two implementation outcomes (adherence to WHO guidelines on PPH prevention and adherence to WHO guidelines on PPH treatment) as critical outcomes. Due to the volume and complexity of the review, we were unable to evaluate in detail the important outcomes, including the rest of the implementation outcomes, to evaluate the full spectrum of implementation. Additionally, we excluded studies that had no comparison group, which may have contributed important insights regarding context‐specific implementation strategies.
The comprehensive ERIC taxonomy of 73 implementation strategies [7] proved to be cumbersome across the variable amount of details we encountered in the included studies. While we applied the nine categories of ERIC implementation strategies [9], we recognized that these nine categories were imperfect and overlapped to some degree. Other studies have critiqued the ERIC taxonomy and offered practical improvements [59, 60], thus we acknowledge that there are other ways of categorizing implementation strategies.
Agreements and disagreements with other studies or reviews
There have been several reviews of PPH prevention, detection, and treatment, demonstrating the tremendous burden of PPH and the importance of finding effective implementation strategies to get evidence‐based therapies into practice. This review builds on and differs from these previous reviews in important ways.
The most recent systematic review, published in 2024, evaluated the effectiveness of care bundles for the prevention and treatment of PPH [22]. It included controlled and uncontrolled studies and reported low certainty around bundles focused on prevention (two controlled studies both among cesarean section births with placental abnormalities) and prevention and treatment combined (one controlled study by Main included in our review), and high certainty around benefits to PPH bundles for treatment for PPH > 500 mL, composite morbidity (PPH > 1000 mL, laparotomy for bleeding, blood transfusion, or death) based exclusively on one controlled trial by Gallos included in our review. Our differences in findings and evidence certainty are largely explained by focusing exclusively on studies that reported results for concurrent control and finding many more controlled studies (13 versus 4).
Multiple reviews have focused on different dimensions of training around obstetric emergencies in general [61, 62, 63, 64]. One systematic review showed that annual obstetric emergency team training had an unclear effect on severe PPH, calling for more controlled trials on optimal team training [65].
Two systematic reviews focusing exclusively on implementation factors are complementary and reinforce the findings of our review. One systematic review focused exclusively on implementation outcomes of policy and program innovations aimed directly at pregnant people, birth attendants, or systems to prevent obstetric hemorrhage in low‐ and middle‐income countries (LMICs) [66] similarly reports important gaps requiring further research and provides complementary insights on implementation factors. Their reported key findings are that: "Greater focus on rigourous evaluation of programmes and policies for prevention (particularly secondary prevention) of [PPH} in low‐ and middle‐income countries (LMICs) is needed, particularly using implementation research methodology to adapt and scale up evidence‐based interventions. Evidence gaps persist on how to develop appropriate policies for implementing effective programmes to prevent [PPH} in LMICs in a context‐specific manner and addressing current knowledge gaps for implementing effective prevention strategies for {PPH in LMICs can decrease negative outcomes." They also report a gap between self‐reported and observed adoption (70.6% versus 21.7%) and geographic variation in acceptability (e.g. high acceptability of self‐administered misoprostol in Uganda and low in Ethiopia), and found no studies of long‐term sustainability. Additionally, they reported implementation barriers such as pervasive myths/fears about uterotonic or other sociocultural barriers, challenges with implementers, health system bottlenecks, and resource limitations. Facilitators included prepared injections or preloaded oxytocin with temperature indicators to increase acceptability and engaging community members early in the introduction, and planning of the intervention to increase acceptability and adoption. While our review differs in that we included experimental and controlled observational studies, focused only on healthcare providers and facilities, and required that studies report on both implementation outcomes and clinical factors, the findings of that review focused exclusively on implementation compliments and reinforce our findings.
The other systematic review, a 2023 Cochrane qualitative review focused on the perceptions and experiences of PPH prevention, detection, and management, describes the broader social, community, and health ecosystems that influence PPH care [18] and found that beliefs around bleeding being "normal" and "expelling impurities"; spiritual beliefs of bleeding as punishment for past mistakes; perceived difficulties among birth attendants for quantitative estimated blood loss; inconsistent availability of resources such as medications, supplies, and blood; workload and exhaustion of providers; and staff turnover may all be contributors to delays in PPH detection and treatment.
Authors' conclusions
Evidence on the effect of implementation strategies for adherence to the World Health Organization (WHO) postpartum hemorrhage (PPH) guidelines and health outcomes is generally of low to very low certainty. Although all the included studies used the implementation strategy of 'train and educate,' the effects seem to be limited when used as a single strategy. Given the heterogeneity of the implementation strategies, how intensely and frequently strategies were used, who the strategies were designed for, and the differing facility settings where the strategies were tested, we are unable to draw any conclusions on the best combination of implementation strategies.
Implications for practice
Multicomponent interventions may improve adherence to WHO PPH prevention recommendations and severe surgical morbidity. The effect of implementation strategies on WHO PPH treatment recommendations is less clear, with studies reporting mixed results. The same study with the same implementation strategy conducted in two different countries had opposite effects (Al‐beity 2019; Hanson 2021), suggesting that context matters. However, the largest, most recent, and most intense implementation study, Gallos 2024, the only study assessed as at low risk of bias, reported a large positive impact on adherence to treatment, PPH ≥ 500 mL, and blood transfusion. There was little or no difference in surgical morbidity in Gallos 2024, possibly due to the restriction of the study population to vaginal births and no surgical component of the clinical intervention.
Robust design and evaluation of implementation strategies in real‐world settings requires both qualitative and quantitative evidence exploring the responsiveness of these strategies to local contextual factors, including site‐specific facilitators and barriers to behavior change. While a qualitative evidence synthesis of implementation strategies is beyond the scope of this review, it is an important area for future practice and research.
Equity‐related implications for practice
The largest burden of maternal mortality from PPH is in low‐resource settings; the majority of studies included in this review were conducted in these environments. The same study conducted in two settings (Al‐beity 2019; Hanson 2021) with different outcomes highlights the need to understand the facility and health system context for optimal implementation approaches. Consistent collection of implementation outcomes (acceptability, perceived appropriateness, feasibility, fidelity, cost, penetration or degree of institutionalization, and sustainability) across settings is needed to ensure equitable reach, uptake, and sustainability of WHO PPH guidelines.
Implications for research
While there is longstanding evidence about which clinical practices are effective for the prevention and treatment of PPH—the leading cause of maternal mortality worldwide—information about how best to translate that knowledge into practice remains a question. The body of evidence included in this review is of low to very low certainty, suggesting that the true effect might be markedly different from the estimated effect. More rigorous studies are required to generate high‐certainty evidence that can inform clinicians, healthcare organizations, and policymakers on how to bridge the know‐do gap from effective practices to effective real‐world implementation and improved health outcomes at scale.
There was a wide variation across studies on which contextual factors (e.g. facility volume, workforce ratios, medication supply availability in intervention and control groups, etc.), and 73 ERIC implementation strategies were reported. It was unusual for studies to thoroughly describe the control group (usual care or enhanced usual care). An ideal target trial would be one where the controls are similar to the intervention group in terms of delivery volume, workforce, patient risk status, medical and technical supplies, surgical capabilities, documentation workflows, and reporting practices. An interdisciplinary panel of experts from implementation science, evidence synthesis, and clinical obstetrics could advance the field by establishing a list of core contextual and implementation measures that should be consistently reported across studies.
Our timeline did not allow us to consider qualitative studies or observational studies without concurrent controls in this review. Similarly, we were not able to review the full set of implementation outcomes. This would be an opportunity for future systematic reviews to provide additional insights. Further robust studies evaluating the impact of implementation strategies to increase uptake of evidence‐based guidelines are vital to maternal health.
Equity‐related implications for research
Postpartum hemorrhage is the leading cause of maternal death, with the most deaths occurring in low‐ and middle‐income countries. Understanding which implementation approaches for evidence‐based guidelines work in which settings is critical to reducing maternal morbidity and mortality while ensuring equitable health outcomes.
Supporting Information
Supplementary materials are available with the online version of this article: 10.1002/14651858.CD016223.
Supplementary materials are published alongside the article and contain additional data and information that support or enhance the article. Supplementary materials may not be subject to the same editorial scrutiny as the content of the article and Cochrane has not copyedited, typeset or proofread these materials. The material in these sections has been supplied by the author(s) for publication under a Licence for Publication and the author(s) are solely responsible for the material. Cochrane accordingly gives no representations or warranties of any kind in relation to, and accepts no liability for any reliance on or use of, such material.
Supplementary material 1 Search strategies
Supplementary material 2 Characteristics of included studies
Supplementary material 3 Characteristics of excluded studies
Supplementary material 4 Characteristics of studies awaiting classification
Supplementary material 5 Characteristics of ongoing studies
Supplementary material 6 Data package
Supplementary material 7 RoB 2 assessments for cluster‐RCTs
Supplementary material 8 ROBINS‐I assessments for NRSIs
New
Additional information
Acknowledgements
We thank Ioannis Gallos and Caitlin Williams from the World Health Organization for their input on our protocol and refinement of our inclusion criteria. We also thank Danielle Tuller and Adam Lindsley from Ariadne Labs for their project management support. We acknowledge Miriam Joseph (French) and Zeev Konstantin G Gurevich (Russian) for their support in translating studies to English.
Jeanne‐Marie Guise and Ethan Litman are employed by Beth Israel Deaconess Medical Center and completed this work as part of that employment. Katherine Semrau and Megan Marx Delaney are employed by Ariadne Labs (Harvard T.H. Chan School of Public Health, Brigham and Women's Hospital) and completed this work as part of that employment. Leslie Choi, Anna Noel‐Storr, and Lindsay Robertson are employed by Cochrane and completed this work as part of that employment.
The following people conducted the editorial process for this commissioned review.
Sign‐off Editor (final editorial decision): Declan Devane, University of Galway
Managing Editor (conducted editorial policy checks, selected peer reviewers, collated peer‐reviewer comments, provided editorial comments/guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments, supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Julia E Moore, The Center for Implementation (clinical/content review); Katharine Shelley, PATH (clinical/content review); Rachel Richardson, Cochrane Evidence Production & Methods Directorate (methods review); Jo Platt, Cochrane Evidence Production & Methods Directorate (search review)
Contributions of authors
KS: review co‐lead (protocol development, study selection, data extraction, data synthesis, GRADE assessment, write‐up).
EL and RM: protocol development, clinical input, study selection, data extraction, data synthesis, risk of bias and GRADE assessments, write‐up.
MMD: study selection, data extraction, data synthesis, risk of bias and GRADE assessments; contributed to write‐up.
LC and LR: study selection, data extraction, risk of bias and GRADE assessments.
ANS: conducted the search; study selection.
JMG: review co‐lead (protocol development, study selection, data extraction, risk of bias and GRADE assessments, data synthesis, write‐up).
Declarations of interest
KS: Brigham and Women's Hospital (Employment); Bill and Melinda Gates Foundation (Grant/Contract)—funding of the BetterBirth trial (The WHO Safe Childbirth Checklist Trial; NCT02148952), grant #OPP1017378; co‐principal investigator/Scientific Director of the BetterBirth trial—this study did not appear in our literature search and thus was not captured by our current review; however, it could become eligible for future updates depending on search parameters. If the BetterBirth trial is included in future review version(s), KS will not make study eligibility decisions about, extract data from, or perform risk of bias or GRADE assessments for the BetterBirth trial.
EL: no relevant interests; Clinical Fellow at Beth Israel Deaconess Medical Center.
RM: no relevant interests; Obstetrician‐Gynecologist, Beth Israel Deaconess Medical Center. If RM becomes involved in any study that could be included in future update(s) of this review, RM will not make study eligibility decisions about, extract data from, or perform risk of bias or GRADE assessments for that study.
MMD: Harvard University School of Public Health (Employment); involved in the BetterBirth trial (The WHO Safe Childbirth Checklist trial; NCT02148952), funded by the Bill and Melinda Gates Foundation (grant #OPP1017378)—this study did not appear in our literature search and thus was not captured by our current review; however, it could become eligible for future update(s) of this review depending on search parameters. If the BetterBirth trial is included in future review version(s), MMD will not make study eligibility decisions about, extract data from, or perform risk of bias or GRADE assessments for the BetterBirth trial.
LC: no relevant interests; Evidence Synthesis Development Editor, Cochrane Central Executive Team, and was not involved in the editorial processing or decision‐making of this review.
LR: no relevant interests; Evidence Synthesis Development Editor, Cochrane Central Executive Team, and was not involved in the editorial processing or decision‐making of this review.
ANS: no relevant interests; Information Specialist, Cochrane Central Executive Team, and was not involved in the editorial processing or decision‐making of this review.
JMG: no relevant interests.
Sources of support
Internal sources
-
Ariadne Labs Discretionary Funding, USA
The funders had no involvement in the development of the protocol or in conducting the review. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of Ariadne Labs.
External sources
-
None, Other
No external sources of support received.
Registration and protocol
Jeanne‐Marie Guise, Katherine Semrau, Rose Molina, Ethan Litman, Megan Marx Delaney. Implementation strategies for WHO guidelines to prevent, diagnose, and manage postpartum hemorrhage: A systematic review. PROSPERO 2024 CRD42024563802 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024563802
Data, code and other materials
As part of the published Cochrane Review, the following is made available for download for users of the Cochrane Library: full search strategies for each database (Supplementary material 1); full citations of each unique report for all studies included (Supplementary material 2), excluded at full‐text screening (Supplementary material 3), awaiting classification (Supplementary material 4), or ongoing (Supplementary material 5), in the final review; study data, including study information, study arms, and study results or test data; consensus risk of bias assessments for cluster‐RCTs (Supplementary material 7) and non‐randomised studies (Supplementary material 8). Appropriate permissions have been obtained for such use. Analyses and data management were conducted within Cochrane’s authoring tool, RevMan, using the inbuilt computation methods [31]. Template data extraction forms from Covidence and Excel are available from the authors on request. The data package is available (Supplementary material 6).
Notes
Published notes in RevMan are for editor use only. Authors should leave this section blank.
References
- 1.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet 2014;2(6):e323-33. [DOI: 10.1016/S2214-109X(14)70227-X] [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. A Roadmap to Combat Postpartum Haemorrhage Between 2023 and 2030. Geneva: World Health Organization, 2023. [Google Scholar]
- 3.Carroli G, Cuesta C, Abalos E, Gulmezoglu A. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice and Research: Clinical Obstetrics and Gynaecology 2008;22(6):999-1012. [DOI: 10.1016/j.bpobgyn.2008.08.004] [DOI] [PubMed] [Google Scholar]
- 4.Lawrence ER, Klein TJ, Beyuo TK. Maternal mortality in low and middle-income countries. Obstetrics and Gynecology Clinics of North America 2022;49(4):713-33. [DOI: 10.1016/j.ogc.2022.07.001] [DOI] [PubMed] [Google Scholar]
- 5.Souza JP. The prevention of postpartum hemorrhage in the community. PLOS Medicine 2013;10:e1001525. [DOI: 10.1371/journal.pmed.1001525] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Trends in Maternal Mortality 2000 to 2020: Estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. Geneva: World Health Organization, 2023. [Google Scholar]
- 7.Powell BJ, Waltz T, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science 2015;10:21. [DOI: 10.1186/s13012-015-0209-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birken SA, Haines ER, Hwang S, Chambers DA, Bunger AC, Nilsen P. Advancing understanding and identifying strategies for sustaining evidence-based practices: a review of reviews. Implementation Science 2020;15(1):88. [DOI: 10.1186/s13012-020-01040-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, Smith JL, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implementation Science 2015;10:109. [DOI: 10.1186/s13012-015-0295-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Everybody's Business: Strengthening Health Systems to Improve Health Outcomes: WHO's Framework for Action. https://iris.who.int/bitstream/handle/10665/43918/9789241596077_eng.pdf.
- 11.Baker R, Camosso‐Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to address determinants of practice. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD005470. [DOI: 10.1002/14651858.CD005470.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Power J, Gilmore B, Vallières F, Toomey E, Mannan H, McAuliffe E. Adapting health interventions for local fit when scaling-up: a realist review protocol. BMJ Open 2019;9(1):e022084. [DOI: 10.1136/bmjopen-2018-022084] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ (Clinical Research Ed.) 2014;348:g1687. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Lasserson T, Thomas J, Flemyng E, Churchill R. Methodological expectations of Cochrane intervention reviews. Cochrane: London, Version August 2023. Available from https://community.cochrane.org/mecir-manual.
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.) 2021;372:n71. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors. https://epoc.cochrane.org/resources/epoc-resources-review-authors.
- 17.World Health Organization. Evaluating the Quality of Care for Severe Pregnancy Complications: the WHO Near-Miss Approach for Maternal Health. Geneva: World Health Organization, 2011. [Google Scholar]
- 18.Akter S, Forbes G, Vazquez CM, Miller S, Althabe F, Coomarasamy A, et al. Perceptions and experiences of the prevention, detection, and management of postpartum haemorrhage: a qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2023, Issue 11. Art. No: CD013795. [DOI: 10.1002/14651858.CD013795.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Administration and Policy in Mental Health 2011;38(2):65-76. [DOI: 10.1007/s10488-010-0319-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implementation Science 2013;8:139. [DOI: 10.1186/1748-5908-8-139] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel JP, Nguyen P, Ramson J, De Silva MS, Pham MD, Sultana S, et al. Effectiveness of care bundles for prevention and treatment of postpartum hemorrhage: a systematic review. American Journal of Obstetrics and Gynecology 2024;231(1):67-91. [DOI: 10.1016/j.ajog.2024.01.012] [DOI] [PubMed] [Google Scholar]
- 23.Covidence. Version accessed 1 May 2024. Melbourne, Australia: Veritas Health Innovation, 2024. Available at covidence.org.
- 24.Microsoft Word. Microsoft Corporation, 2024. Available from https://office.microsoft.com/word.
- 25.Microsoft Excel. Version 2405. Microsoft Corporation, 2024. Available from https://office.microsoft.com/excel.
- 26.Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (updated August 2024). Cochrane, 2024. Available from www.training.cochrane.org/handbook.
- 28.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2021;12(1):55-61. [DOI: 10.1002/jrsm.1411] [DOI] [PubMed] [Google Scholar]
- 29.Cochrane Pregnancy and Childbirth Group. Identifying and handling potentially untrustworthy trials—Trustworthiness Screening Tool (TST). Version 3.0. Cochrane; 2023. https://www.cochranelibrary.com/cdsr/editorial-policies/problematic-studies-implementation-guidance.
- 30.Page MJ, Higgins JP, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.3.
- 31.Review Manager (RevMan). Version 8.1.1. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
- 32.Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from https://gdt.gradepro.org/app/handbook/handbook.html.
- 33.GRADEpro GDT. Version accessed 28 August 2024. Hamilton (ON): McMaster University (developed by Evidence Prime), 2024. Available at https://www.gradepro.org.
- 34.Alwy Al-beity F, Pembe A, Hirose A, Morris J, Leshabari S, Marrone G, et al. Effect of the competency-based helping mothers survive bleeding after birth (HMS BAB) training on maternal morbidity: a cluster-randomised trial in 20 districts in Tanzania. BMJ Global Health 2019;4(2):e001214. [DOI: 10.1136/bmjgh-2018-001214] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Althabe F, Buekens P, Bergel E, Belizan JM, Campbell MK, Moss N, et al, For the Guidelines Trial Group. A behavioral intervention to improve obstetrical care. New England Journal of Medicine 2008;358(18):1929-40. [DOI: 10.1056/NEJMsa071456] [DOI] [PubMed] [Google Scholar]
- 36.Deneux-Tharaux C, Dupont C, Colin C, Rabilloud M, Touzet S, Lansac J, et al. Multifaceted intervention to decrease the rate of severe postpartum haemorrhage: the PITHAGORE6 cluster-randomised controlled trial. BJOG 2010;117(10):1278-87. [DOI: 10.1111/j.1471-0528.2010.02648.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans CL, Bazant E, Atukunda I, Williams E, Niermeyer S, Hiner C, et al. Peer-assisted learning after onsite, low-dose, high-frequency training and practice on simulators to prevent and treat postpartum hemorrhage and neonatal asphyxia: a pragmatic trial in 12 districts in Uganda. PLOS ONE 2018;13(12):e0207909. [DOI: 10.1371/journal.pone.0207909] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallos I, Devall A, Martin J, Middleton L, Beeson L, Galadanci H, et al. Randomized trial of early detection and treatment of postpartum hemorrhage. Obstetrical & Gynecological Survey 2024;79(1):9-11. [DOI: 10.1097/01.ogx.0001006884.97006.bc] [DOI] [Google Scholar]
- 39.Hanson C, Atuhairwe S, Lucy Atim J, Marrone G, Morris JL, Kaharuza F. Effects of the Helping Mothers Survive Bleeding after Birth training on near miss morbidity and mortality in Uganda: a cluster-randomized trial. International Journal of Gynaecology and Obstetrics 2021;152(3):386-94. [DOI: 10.1002/ijgo.13395] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klokkenga CM, Enemark U, Adanu R, Lund S, Sorensen BL, Attermann J. The effect of smartphone training of Ghanaian midwives by the Safe Delivery application on the incidence of postpartum hemorrhage: a cluster randomised controlled trial. Cogent Medicine 2019;6(1):1632016. [DOI: 10.1080/2331205X.2019.1632016] [DOI] [Google Scholar]
- 41.Liabsuetrakul T, Palanukunwong K, Chinduereh A, Oumudee N. Evaluation of a multifaceted postpartum hemorrhage-management intervention in community hospitals in Southern Thailand. International Journal of Gynaecology and Obstetrics 2017;139(1):39-44. [DOI: 10.1002/ijgo.12253] [DOI] [PubMed] [Google Scholar]
- 42.Main EK, Cape V, Abreo A, Vasher J, Woods A, Carpenter A, et al. Reduction of severe maternal morbidity from hemorrhage using a state perinatal quality collaborative. American Journal of Obstetrics and Gynecology 2017;216(3):298-e1. [DOI] [PubMed] [Google Scholar]
- 43.Mogilevkina I, Gurianov V, Lindmark G. Effectiveness of emergency obstetric care training at the regional level in Ukraine: a non-randomized controlled trial. BMC Pregnancy and Childbirth 2022;22(1):145. [DOI: 10.1186/s12884-022-04458-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naidoo M, Moodley J, Gathiram P, Sartorius B. The impact of a modified World Health Organization surgical safety checklist on maternal outcomes in a South African setting: a stratified cluster-randomised controlled trial. South African Medical Journal 2017;107(3):248-57. [DOI: 10.7196/SAMJ.2017.v107i3.11320] [DOI] [PubMed] [Google Scholar]
- 45.Sloan NL, Nguyen TN, Do TH, Quimby C, Winikoff B, Fassihian G. Effectiveness of lifesaving skills training and improving institutional emergency obstetric care readiness in Lam Dong, Vietnam. Journal of Midwifery & Women's Health 2005;50(4):315-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 46.de Ven J, Fransen AF, Schuit E, Runnard Heimel PJ, Mol BW, Oei SG. Does the effect of one-day simulation team training in obstetric emergencies decline within one year? A post-hoc analysis of a multicentre cluster randomised controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;216:79-84. [DOI: 10.1016/j.ejogrb.2017.07.020] [DOI] [PubMed] [Google Scholar]
- 47.ISRCTN17679951. Effectiveness of a maternity improvement programme to reduce excess bleeding and need for transfusion after childbirth: the Obstetric Bleeding Study UK (OBS UK). https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN17679951 2023.
- 48.JRCT1090220328. An effectiveness study of a continuous approach from routine intrapartum care to emergency obstetric care on reducing severe maternal outcomes through nation-wide rollout in Lao PDR. https://jrct.niph.go.jp/latest-detail/jRCT1090220328 2018.
- 49.Kamala BA, Ersdal HL, Mduma E, Moshiro R, Girnary S, Østrem OT, et al. SaferBirths bundle of care protocol: a stepped-wedge cluster implementation project in 30 public health-facilities in five regions, Tanzania. BMC Health Services Research 2021;21:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayanna K, Bradley J, Mony P, Cunningham T, Washington M, Bhat S, et al. Effectiveness of onsite nurse mentoring in improving quality of institutional births in the primary health centres of high priority districts of karnataka, south India: a cluster randomized trial. PloS One 2016;11(9):e0161957. [DOI: 10.1371/journal.pone.0161957] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Althabe F, Buekens P, Bergel E, Belizan JM, Kropp N, Wright L, et al, For the Guidelines Trial Group. A cluster randomized controlled trial of a behavioral intervention to facilitate the development and implementation of clinical practice guidelines in Latin American maternity hospitals: the Guidelines Trial: Study protocol [ISRCTN82417627]. BMC Women's Health 2005;5(1):4. [DOI: 10.1186/1472-6874-5-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanson C, Pembe AB, Alwy F, Atuhairwe S, Leshabari S, Morris J; The HMS BAB study team. Evaluating the effect of the Helping Mothers Survive Bleeding After Birth (HMS BAB) training in Tanzania and Uganda: study protocol for a randomised controlled trial. Trials 2017;18(1):307. [DOI: 10.1186/s13063-017-2056-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klokkenga CM. An Investigation of Smartphone Application in Emergency Obstetric Care in Ghana: a Cluster Randomised Trial (PhD thesis). Aarhus University, 2017. [ISSN: 0908-2131] [Google Scholar]
- 54.Forbes G, Akter S, Miller S, Galadanci H, Qureshi Z, Al-beity Fadhlun Alwy, et al. Development and piloting of implementation strategies to support delivery of a clinical intervention for postpartum hemorrhage in four sub-saharan Africa countries. Global Health: Science and Practice 2024;12(5):e2300387. [DOI: 10.9745/GHSP-D-23-00387] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams E, Bazant ES, Holcombe S, Atukunda I, Namugerwa RI, Britt K, et al. “Practice so that the skill does not disappear”: mixed methods evaluation of simulator-based learning for midwives in Uganda. Human Resources for Health 2019;17(1):24. [DOI: 10.1186/s12960-019-0350-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Ven J, Baaren GJ, Fransen AF, Runnard Heimel PJ, Mol BW, Oei SG. Cost-effectiveness of simulation-based team training in obstetric emergencies (TOSTI study). European Journal of Obstetrics and Gynecology and Reproductive Biology 2017;216:130-7. [DOI: 10.1016/j.ejogrb.2017.07.027] [DOI] [PubMed] [Google Scholar]
- 57.Wiesehan EC, Keesara SR, Krissberg JR, Main EK, Goldhaber-Fiebert JD. State perinatal quality collaborative for reducing severe maternal morbidity from hemorrhage: a cost-effectiveness analysis. Obstetrics and Gynecology 2023;141(2):387-94. [DOI: 10.1097/AOG.0000000000005060] [DOI] [PubMed] [Google Scholar]
- 58.Williams EV, Goranitis I, Oppong R, Perry SJ, Devall AJ, Martin JT, et al. A cost-effectiveness analysis of early detection and bundled treatment of postpartum hemorrhage alongside the E-MOTIVE trial. Nature Medicine 2024;30(8):2343-8. [DOI: 10.1038/s41591-024-03069-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry CK, Damschroder LJ, Hemler JR, Woodson TT, Ono SS, Cohen DJ. Specifying and comparing implementation strategies across seven large implementation interventions: a practical application of theory. Implementation Science 2019;14(1):32. [DOI: 10.1186/s13012-019-0876-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yakovchenko V, Chinman M, Lamorte C, Powell BJ, Waltz TJ, Merante M, et al. Refining Expert Recommendations for Implementing Change (ERIC) strategy surveys using cognitive interviews with frontline providers. Implementation Science Communications 2023;4(1):42. [DOI: 10.1186/s43058-023-00409-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ameh CA, Mdegela M, White S, den Broek N. The effectiveness of training in emergency obstetric care: a systematic literature review. Health Policy and Planning 2019;34(4):257-70. [DOI: 10.1093/heapol/czz028] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brogaard L, Kierkegaard O, Hvidman L, Jensen KR, Musaeus P, Uldbjerg N, et al. The importance of non-technical performance for teams managing postpartum haemorrhage: video review of 99 obstetric teams. British Journal of Obstetrics and Gynaecology 2019;126(8):1015-23. [DOI: 10.1111/1471-0528.15655] [DOI] [PubMed] [Google Scholar]
- 63.Mendez-Figueroa H, Bell CS, Wagner SM, Pedroza C, Gupta M, Mulder I, et al. Postpartum hemorrhage drills or simulations and adverse outcomes: a systematic review and Bayesian meta-analysis. Journal of Maternal-Fetal & Neonatal Medicine 2022;35(26):10416-27. [DOI: 10.1080/14767058.2022.2128659] [DOI] [PubMed] [Google Scholar]
- 64.Merien AE, de Ven J, Mol BW, Houterman S, Oei SG. Multidisciplinary team training in a simulation setting for acute obstetric emergencies: a systematic review. Obstetrics and Gynecology 2010;115(5):1021-31. [DOI: 10.1097/AOG.0b013e3181d9f4cd] [DOI] [PubMed] [Google Scholar]
- 65.Brogaard L, Glerup Lauridsen K, Lofgren B, Krogh K, Paltved C, Boie S, et al. The effects of obstetric emergency team training on patient outcome: A systematic review and meta-analysis. Acta Obstetricia et Gynecologica Scandinavica 2022;101(1):25-36. [DOI: 10.1111/aogs.14263] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryan N, Vieira D, Goffman D, Bloch EM, Akaba GO, D'mello BS, et al. Implementation outcomes of policy and programme innovations to prevent obstetric haemorrhage in low- and middle-income countries: a systematic review. Health Policy and Planning 2020;35(9):1208-27. [DOI: 10.1093/heapol/czaa074] [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization. WHO Recommendations: Uterotonics for the Prevention of Postpartum Haemorrhage. Geneva: World Health Organization, 2018. [PubMed] [Google Scholar]
- 68.World Health Organization. WHO Recommendations: Intrapartum Care for a Positive Childbirth Experience. Geneva: World Health Organization, 2018. [PubMed] [Google Scholar]
- 69.World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
- 70.World Health Organization. WHO Recommendations on the Assessment of Postpartum Blood Loss and Use of a Treatment Bundle for Postpartum Haemorrhage. Geneva: World Health Organization, 2023. [PubMed] [Google Scholar]
- 71.World Health Organization. Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage; Highlights and Key Messages from the World Health Organization’s 2017 Global Recommendation; October 2017. Geneva: World Health Organization 2017;WHO/RHR/17.21:1-5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Search strategies
Supplementary material 2 Characteristics of included studies
Supplementary material 3 Characteristics of excluded studies
Supplementary material 4 Characteristics of studies awaiting classification
Supplementary material 5 Characteristics of ongoing studies
Supplementary material 6 Data package
Supplementary material 7 RoB 2 assessments for cluster‐RCTs
Supplementary material 8 ROBINS‐I assessments for NRSIs
Data Availability Statement
As part of the published Cochrane Review, the following is made available for download for users of the Cochrane Library: full search strategies for each database (Supplementary material 1); full citations of each unique report for all studies included (Supplementary material 2), excluded at full‐text screening (Supplementary material 3), awaiting classification (Supplementary material 4), or ongoing (Supplementary material 5), in the final review; study data, including study information, study arms, and study results or test data; consensus risk of bias assessments for cluster‐RCTs (Supplementary material 7) and non‐randomised studies (Supplementary material 8). Appropriate permissions have been obtained for such use. Analyses and data management were conducted within Cochrane’s authoring tool, RevMan, using the inbuilt computation methods [31]. Template data extraction forms from Covidence and Excel are available from the authors on request. The data package is available (Supplementary material 6).