Abstract
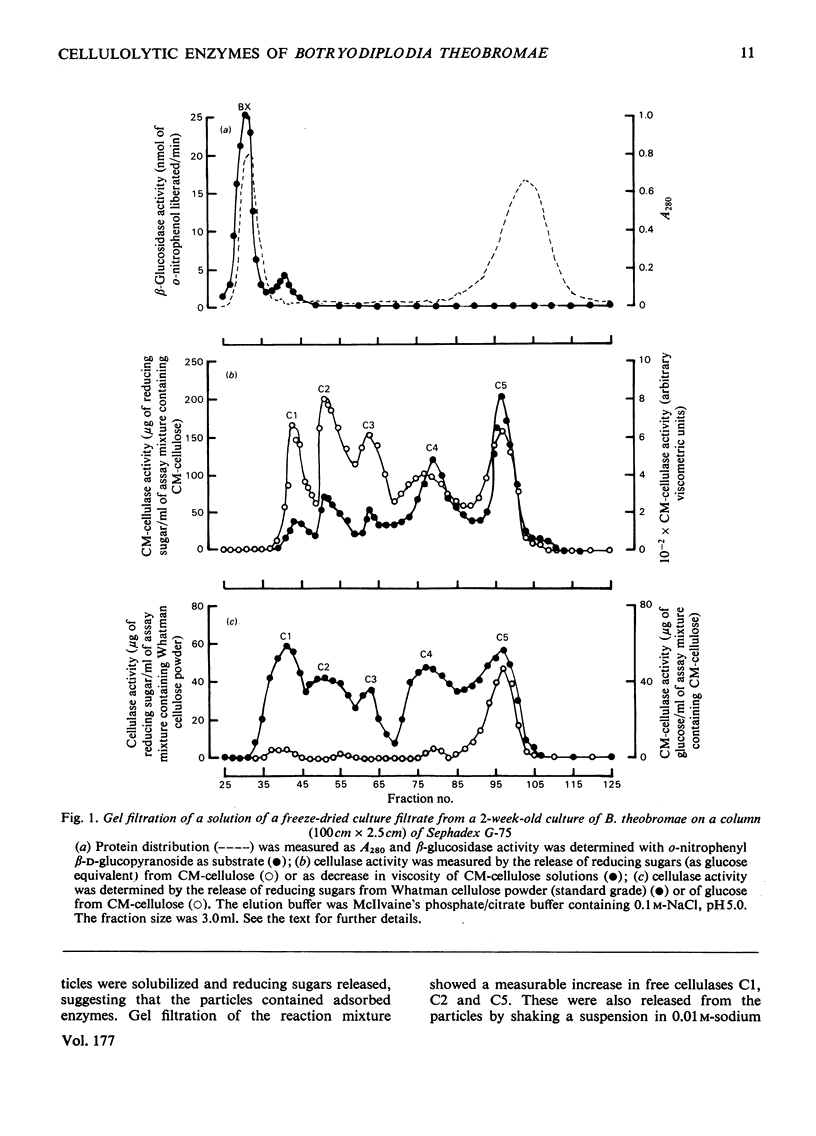
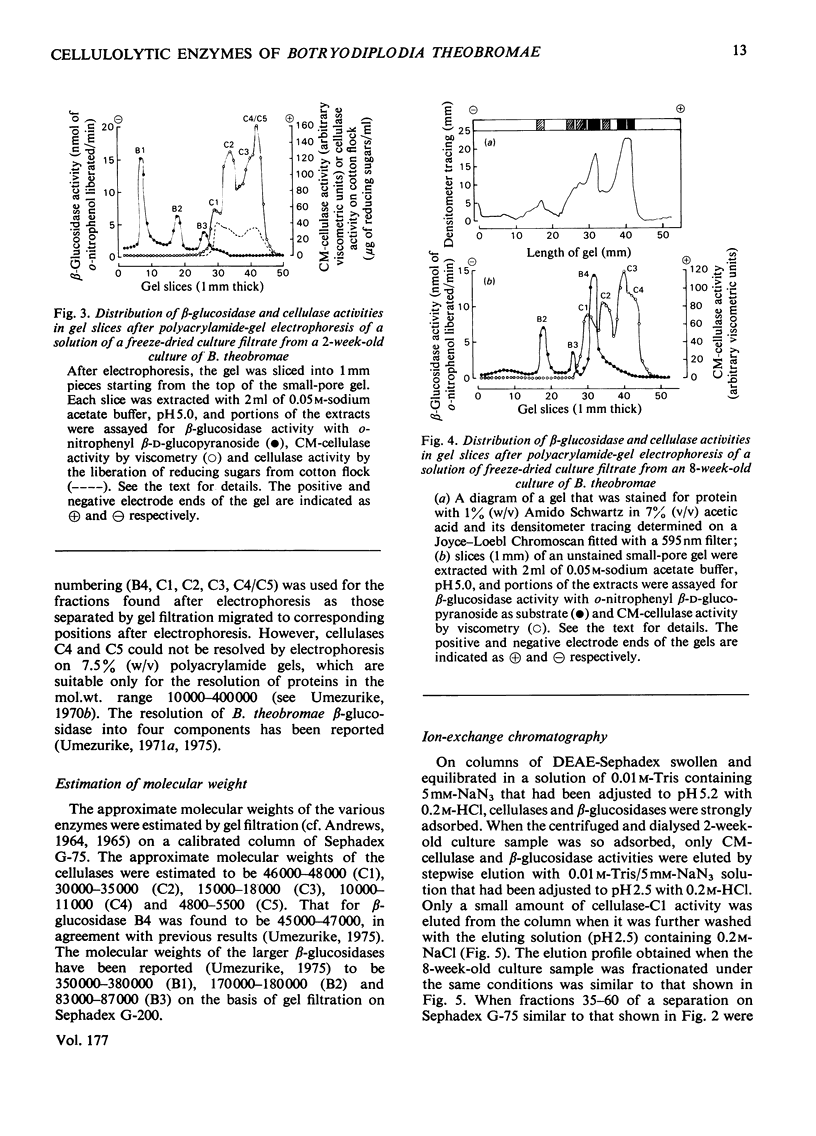
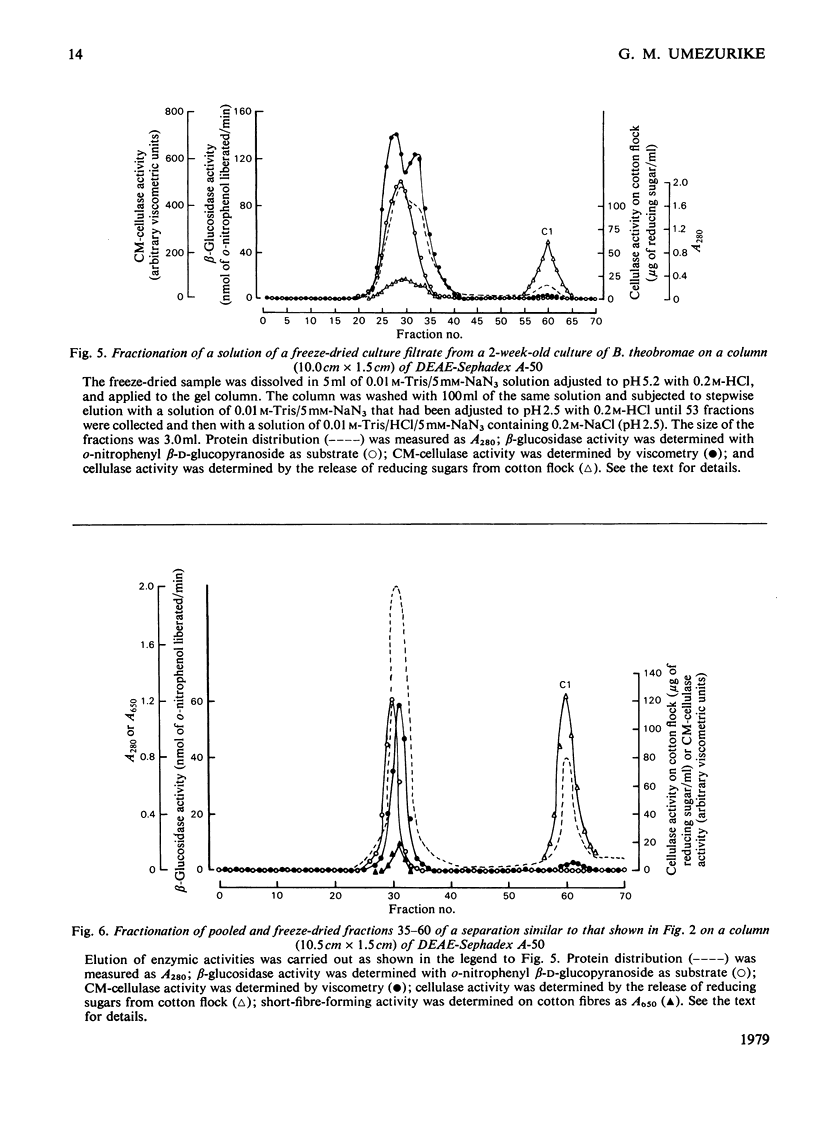
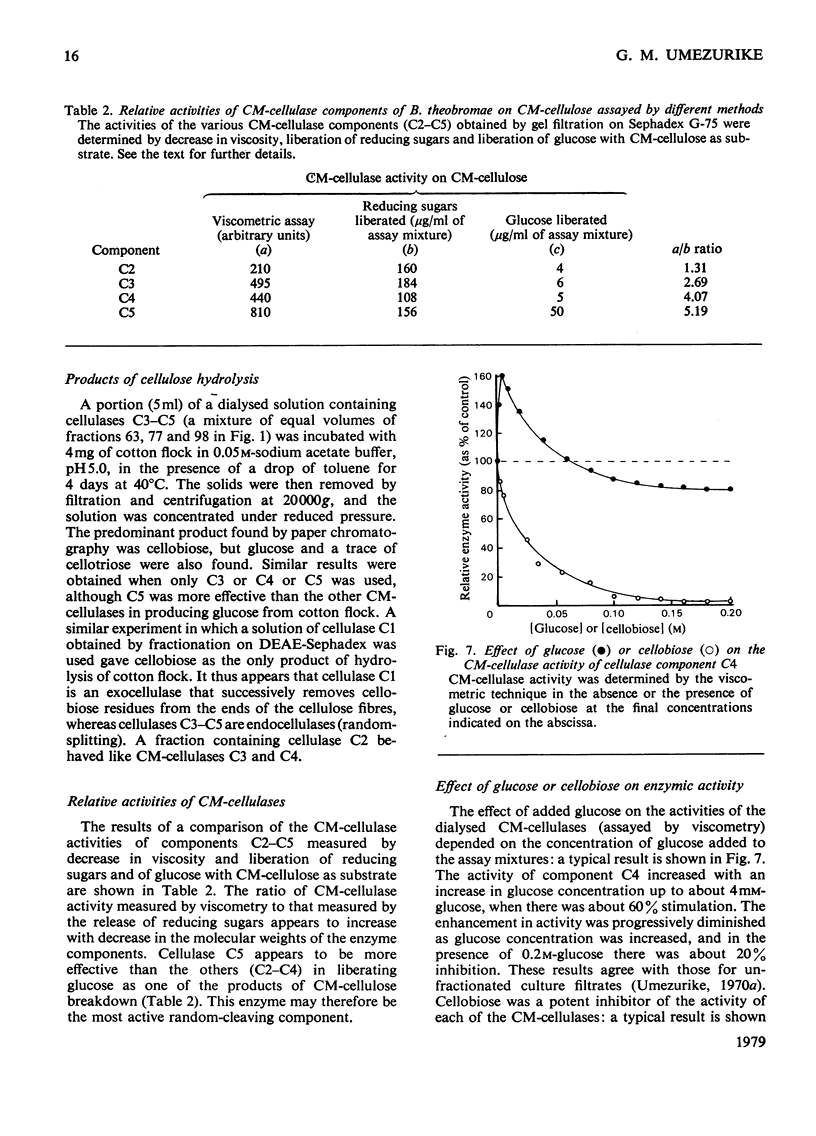
1. Filtrates from cultures of different ages of Botryodiplodia theobromae Pat. were fractionated by gel filtration, ion-exchange chromatography and polyacrylamide-gel electrophoresis. 2. Five cellulases (C1, C2, C3, C4 and C5) were found, and their molecular weights, estimated by gel filtration, were 46000–48000 (C1), 30000–35000 (C2), 15000–18000 (C3), 10000–11000 (C4) and 4800–5500 (C5). 3. Cellulase C5 was absent from old culture filtrates. 4. Cellulase C1 had little or no activity on CM-cellulose (viscometric assay), but degraded cotton flock and Whatman cellulose powder to give cellobiose only. 5. The other components (C2–C5) produced cellobiose and smaller amounts of glucose and cellotriose from cellulosic substrates and were more active in lowering the viscosity of CM-cellulose. 6. The ratio of activities assayed by viscometry and by the release of reducing sugars from CM-cellulose increased with decrease in the molecular weights of cellulases C2–C5. 7. Cellobiose inhibited the activities of the cellulases, but glucose stimulated at low concentrations although it inhibited at high concentrations. 8. A high-molecular-weight β-glucosidase (component B1, mol.wt. 350000–380000) predominated in filtrates from young cultures, but a low-molecular-weight enzyme (B4, mol.wt. 45000–47000) predominated in older filtrates. 9. Intermediate molecular species of β-glucosidase (B2, mol.wt. 170000–180000; B3, mol.wt. 83000–87000) were also found. 10. Cellulases C2–C5 acted in synergism with C1, particularly in the presence of β-glucosidase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghem L. E., Pettersson L. G. The mechanism of enzymatic cellulose degradation. Purification of a cellulolytic enzyme from Trichoderma viride active on highly ordered cellulose. Eur J Biochem. 1973 Aug 1;37(1):21–30. doi: 10.1111/j.1432-1033.1973.tb02952.x. [DOI] [PubMed] [Google Scholar]
- GILLIGAN W., REESE E. T. Evidence for multiple components in microbial cellulases. Can J Microbiol. 1954 Oct;1(2):90–107. doi: 10.1139/m55-013. [DOI] [PubMed] [Google Scholar]
- Halliwell G., Griffin M. The nature and mode of action of the cellulolytic component C1 of Trichoderma koningii on native cellulose. Biochem J. 1973 Dec;135(4):587–594. doi: 10.1042/bj1350587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell G., Riaz M. The formation of short fibres from native cellulose by components of Trichoderma koningii cellulase. Biochem J. 1970 Jan;116(1):35–42. doi: 10.1042/bj1160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOIMAN P., ROELOFSEN P. A., SWEERIS S. Some properties of cellulase from Myrothecium verrucaria. Enzymologia. 1953 Dec 30;16(4):237–246. [PubMed] [Google Scholar]
- Li L. H., Flora R. M., King K. W. Individual roles of cellulase components derived from Trichoderma viride. Arch Biochem Biophys. 1965 Aug;111(2):439–447. doi: 10.1016/0003-9861(65)90207-9. [DOI] [PubMed] [Google Scholar]
- PETTERSSON G., COWLING E. B., PORATH J. Studies on celluloytic enzymes. I. Isolation of a low-molecular-weight cellulase from Polyporus versicolor. Biochim Biophys Acta. 1963 Jan 8;67:1–8. doi: 10.1016/0006-3002(63)91791-8. [DOI] [PubMed] [Google Scholar]
- PETTERSSON G., PORATH J. Studies on cellulolytic enzymes. II. Multiplicity of the cellulolytic enzymes of Polyporus versicolor. Biochim Biophys Acta. 1963 Jan 8;67:9–15. doi: 10.1016/0006-3002(63)91792-x. [DOI] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELBY K., MAITLAND C. C. THE FRACTIONATION OF MYROTHECIUM VERRUCARIA CELLULASE BY GEL FILTRATION. Biochem J. 1965 Mar;94:578–583. doi: 10.1042/bj0940578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORVICK W. O., KING K. W. The complexity and mode of action of the cellulase system of Cellvibrio gilvus. J Biol Chem. 1960 Feb;235:303–307. [PubMed] [Google Scholar]
- Selby K., Maitland C. C. The cellulase of Trichoderma viride. Separation of the components involved in the solubilization of cotton. Biochem J. 1967 Sep;104(3):716–724. doi: 10.1042/bj1040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby K., Maitland C. C., Thompson K. V. The degradation of cotton cellulose by the extracellular cellulase of Myrothecium verrucaria. 2. The existence of an ;exhaustible' cellulase. Biochem J. 1963 Aug;88(2):288–296. doi: 10.1042/bj0880288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezurike G. M. Kinetic properties of -glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Oct;250(1):182–191. doi: 10.1016/0005-2744(71)90132-x. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The purification and properties of extracellular beta-glucosidase from Botryodiplodia theobromae Pat. Biochim Biophys Acta. 1971 Feb 10;227(2):419–428. doi: 10.1016/0005-2744(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The subunit structure of beta-glucosidase from Botryodiplodia theobromae Pat. Biochem J. 1975 Feb;145(2):361–368. doi: 10.1042/bj1450361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITAKER D. R., COLVIN J. R., COOK W. H. The molecular weight and shape of Myrothecium verrucaria cellulase. Arch Biochem Biophys. 1954 Apr;49(2):257–262. doi: 10.1016/0003-9861(54)90195-2. [DOI] [PubMed] [Google Scholar]
- WHITAKER D. R. Purification of Myrothecium verrucaria cellulase. Arch Biochem Biophys. 1953 Apr;43(2):253–268. doi: 10.1016/0003-9861(53)90120-9. [DOI] [PubMed] [Google Scholar]
- Whitney P., Chapman J. M., Heale J. B. Carboxymethylcellulase production by Verticillium albo-atrum. J Gen Microbiol. 1969 May;56(2):215–225. doi: 10.1099/00221287-56-2-215. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem J. 1972 Aug;128(5):1183–1192. doi: 10.1042/bj1281183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M. The cellulase of Fusarium solani. Purification and specificity of the -(1-4)-glucanase and the -D-glucosidase components. Biochem J. 1971 Feb;121(3):353–362. doi: 10.1042/bj1210353. [DOI] [PMC free article] [PubMed] [Google Scholar]


