Abstract
1. A trypsin inhibitor from the tick Boophilus microplus was purified by ion-exchange chromatography and gel filtration. 2. It is pure by the criteria of constant specific activity on gel filtration and by electrophoresis on polyacrylamide gels containing sodium dodecyl sulphate. 3. The protein undergoes reversible polymerization, dissociating at low pH. 4. The apparent molecular weight measured by electrophoresis on polyacrylamide gels containing sodium dodecyl sulphate is 18,500. 5. Inhibition of trypsin occurs by formation of a 1 :1 molar complex. 6. Chymotrypsin is also inhibited, though the dissociation constant of the complex formed is larger than with trypsin. The protein possesses independent sites for the inhibition of chymotrypsin and trypsin. 7. The inhibitor preparation gives an immediate hypersensitivity reaction on intradermal injection into cattle that have been exposed to the tick. The allergenic activity is due to the inhibitor protein itself and not to contaminating material, since the two activities were not separated during purification or in two subsequent affinity-chromatography procedures. 8. The hypersensitivity reaction is a true immunological response, since it is found in almost all cattle that have been exposed to the tick, but not in unexposed animals. In addition, passive cutaneous anaphylaxis can be demonstrated with serum from exposed, but not from unexposed, animals.
Full text
PDF
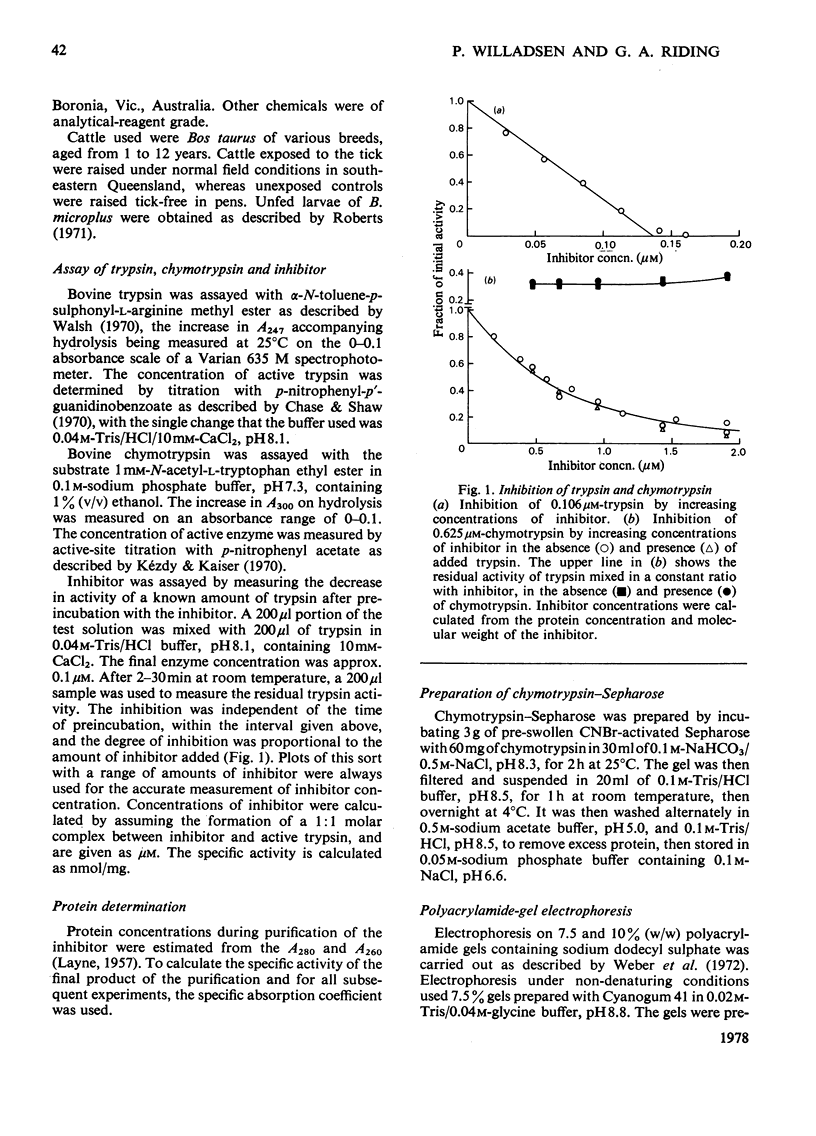

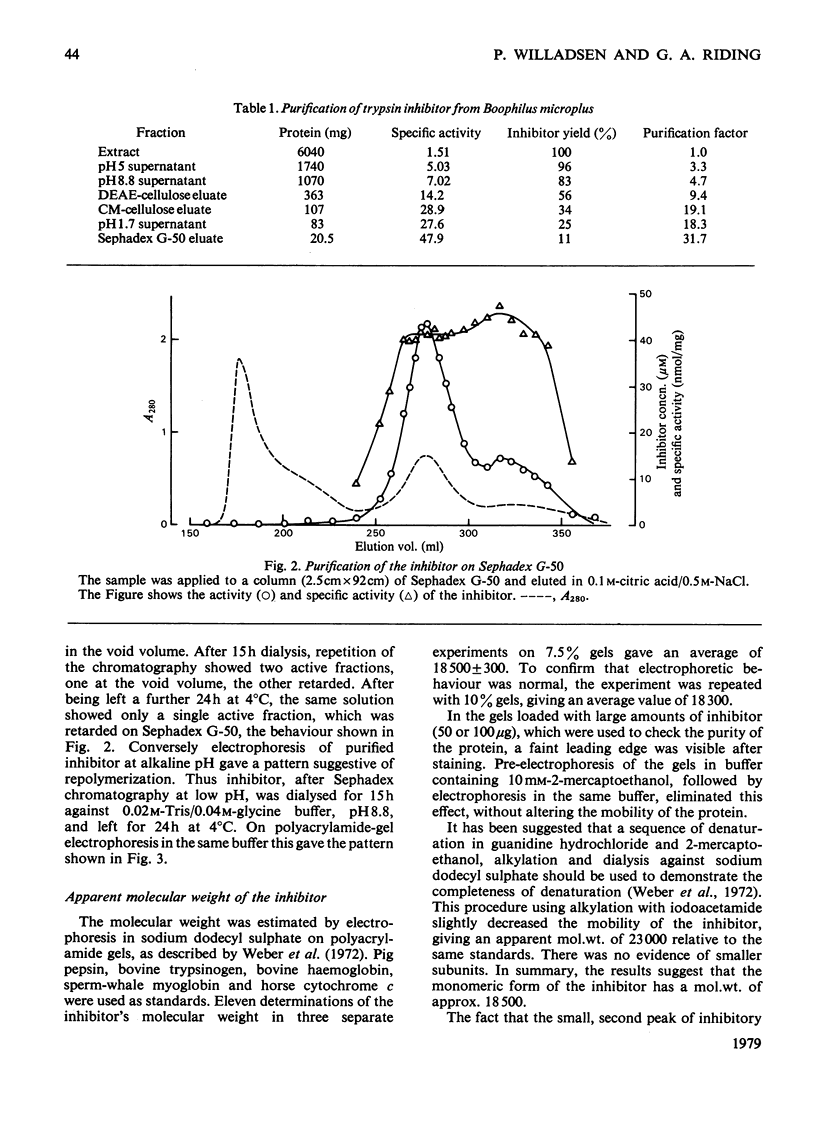
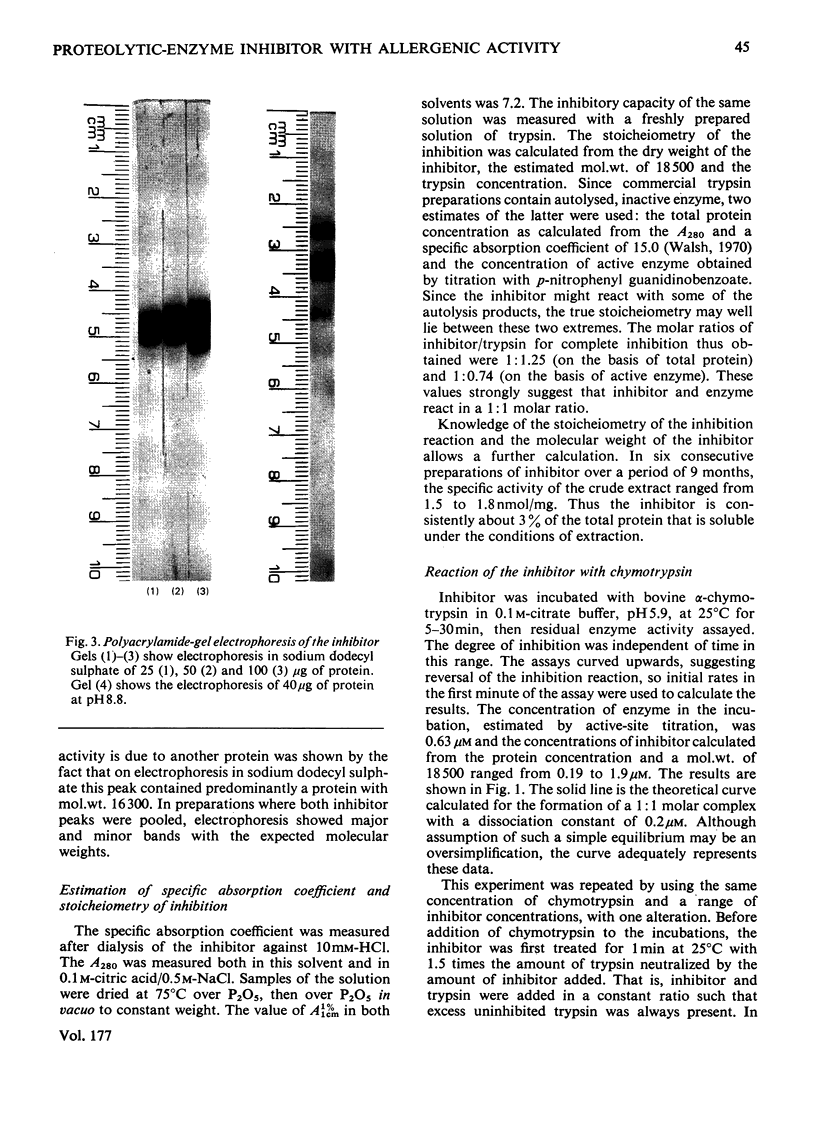

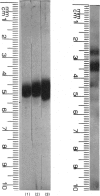
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birk Y. Chemistry and nutritional significance of proteinase inhibitors from plant sources. Ann N Y Acad Sci. 1968 Jun 28;146(2):388–399. doi: 10.1111/j.1749-6632.1968.tb20299.x. [DOI] [PubMed] [Google Scholar]
- Fritz H., Hochstrasser K. Proteinase (elastase) inhibitors from dog submandibular glands. Methods Enzymol. 1976;45:860–869. doi: 10.1016/s0076-6879(76)45079-6. [DOI] [PubMed] [Google Scholar]
- Krahn J., Stevens F. C. Lima bean trypsin inhibitor. Limited proteolysis by trypsin and chymotrypsin. Biochemistry. 1970 Jun 23;9(13):2646–2652. doi: 10.1021/bi00815a013. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Rothwell T. L., Bremner K. C., Schnitzerling H. J., Nolan J., Keith R. K. Acetylcholinesterase secretion by parasitic nematodes. I. Evidence for secretion of the enzyme by a number of species. Int J Parasitol. 1973 Sep;3(5):589–597. doi: 10.1016/0020-7519(73)90083-0. [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Romanowski R. D. The secretory nature of the excretory gland cells of Strephanurus dentatus. 3. Proteinase inhibitors. Exp Parasitol. 1974 Jun;35(3):363–368. doi: 10.1016/0014-4894(74)90041-1. [DOI] [PubMed] [Google Scholar]
- Roberts J. A. Behavior of larvae of the cattle tick, Boophilus microplus (Canestrini), on cattle of differing degrees of resistance. J Parasitol. 1971 Jun;57(3):651–656. [PubMed] [Google Scholar]
- Rothwell T. L., Merritt G. C. Acetylcholinesterase secretion by parasitic nematodes. IV. Antibodies against the enzyme in Trichostrongylus colubriformis infected sheep. Int J Parasitol. 1974 Feb;4(1):63–71. doi: 10.1016/0020-7519(74)90010-1. [DOI] [PubMed] [Google Scholar]
- Tomimatsu Y., Clary J. J., Bartulovich J. J. Physical characterization of ovoinhibitor, a trypsin and chymotrypsin inhibitor from chicken egg white. Arch Biochem Biophys. 1966 Sep 9;115(3):536–544. doi: 10.1016/0003-9861(66)90073-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Williams P. G. Isolation and partial characterization of an antigen from the cattle tick, Boophilus microplus. Immunochemistry. 1976 Jul;13(7):591–597. doi: 10.1016/0019-2791(76)90171-3. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Williams P. G., Roberts J. A., Kerr J. D. Responses of cattle to allergens from Boophilus microplus. Int J Parasitol. 1978 Apr;8(2):89–95. doi: 10.1016/0020-7519(78)90003-6. [DOI] [PubMed] [Google Scholar]