Abstract
Background
Estimated glucose disposal rate (eGDR) is considered as a reliable alternative indicator of insulin resistance. However, the relationship between eGDR levels and mortality among individuals with cardiometabolic syndrome (CMS), as well as within different glucose metabolic states in this population, remains unclear.
Methods
We conducted a cohort study on 9928 CMS participants from the National Health and Nutrition Examination Survey (NHANES) database from 1999 to 2018. The relationship between eGDR levels and mortality in the CMS population was evaluated using multivariable Cox proportional hazards regression models and restricted cubic splines (RCS). Finally, stratified analysis was performed to determine the relationship between eGDR levels and mortality in different subgroups.
Results
Cox regression analysis showed a significant correlation between eGDR levels and both all-cause and cause-specific mortality in the entire CMS population (all p < 0.05). RCS analysis revealed a non-linear relationship between eGDR levels and both all-cause (p for overall < 0.001, p for non-linear < 0.001) and diabetes specific mortality (p for overall < 0.001, p for non-linear = 0.004) in CMS population, while a linear relationship with cardiovascular specific mortality (p for overall < 0.001, p for non-linear = 0.091). In participants with baseline diabetes mellitus (DM), eGDR levels were significantly correlated with all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality (all p < 0.05). In CMS participants with baseline pre-diabetes mellitus (Pre-DM), eGDR levels were significantly correlated with cardiovascular-specific and diabetes-specific mortality (all p < 0.05). In CMS participants with baseline normal glucose regulation (NGR), eGDR levels were only significantly related to diabetes specific mortality (p < 0.05).
Conclusion
There is a significant correlation between eGDR levels and both all-cause and cause-specific mortality in the entire CMS population. Furthermore, the protective effect of high eGDR levels on mortality persists across various glucose metabolic states.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-025-01636-5.
Keywords: Cardiometabolic syndrome, Estimated glucose disposal rate, All-cause and cause specific mortality, Glucose metabolism states
Background
The increasing global prevalence of cardiometabolic syndrome (CMS) has emerged as a significant public health concern, contributing significantly to mortality and disability on a global scale [1, 2]. CMS is comprised of a series of metabolic dysfunctions, including insulin resistance (IR), hypertension, central obesity, lipid abnormalities, and other risk factors [3, 4]. IR is a significant factor leading to adverse events in CMS, interacting with other metabolic risk factors to promote various metabolic diseases and increase the risk of mortality [5–7]. Currently, hyperinsulinemic-euglycemic clamp is considered the gold standard for diagnosing IR, but its invasiveness and high cost limit its clinical application [8, 9]. Therefore, the search for simple, convenient, and easily applicable indicators for assessing IR is crucial, such as homeostasis model assessment of insulin resistance (HOMA-IR), triglyceride glucose index (TyG index), estimated glucose disposal rate (eGDR), which are important non-invasive assessment indicators [10–12].
eGDR is an assessment method different from traditional IR indicators, integrating waist circumference(WC), hypertension, and Hemoglobin A1c (HbA1c) to comprehensively evaluate IR. Interestingly, the components included in eGDR, namely WC, hypertension, and HbA1c, are also diagnostic elements of CMS. Several studies have shown that eGDR can effectively predict diabetes-related complications such as nephropathy, neuropathy, and retinopathy [13–15]. In addition, eGDR has the ability to predict mortality and has good predictive value for cardiovascular events and mortality in type 2 diabetes mellitus (T2DM) and non-diabetic patients [12, 16–19]. However, there is currently a lack of in-depth research on the relationship between eGDR and mortality in population with CMS.
Our study aims to explore the relationship between eGDR and both all-cause and cause-specific mortality in population with CMS through a large-scale prospective cohort study in the United States, and further investigate the role of eGDR in population with CMS in different glucose metabolic state.
Methods
Study population and design
The data for this study is from the National Health and Nutrition Examination Survey (NHANES) database. We downloaded research data from 10 interview cycles spanning from 1999 to 2018, with a total of 101,316 participants undergoing examination. We excluded participants under the age of 18 (42,112 participants) and further excluded participants with missing CMS diagnostic-related indicators and lipid-related data (35,707 participants). After merging with mortality data, a total of 9928 participants were diagnosed with CMS, with 4682 males and 5246 females included in this study. The diagnosis of CMS refers to the National Cholesterol Education Program (NCEP): Adult Treatment Panel (ATP) III [20]. For detailed inclusion criteria, please refer to Fig. 1. The dataset used for the current study and analysis can be accessed at any time on the NHANES official website.
Fig. 1.
Procedure of subject screening and diagnosis in this study. NHANES: National Health and Nutrition Examination Survey; CMS: cardiometabolic syndrome; FBG: fasting blood glucose; HDL-C: high-density lipoprotein cholesterol; TG: triglycerides
Assessment of covariates
The NHANES database provided data on diverse demographic and health-related factors, encompassing the age, gender, race, education level (below high school, high school and above), marital status (married, unmarried), poverty-income ratio (PIR) (PIR < 1.3 indicating poverty), smoking status (never, former, current), alcohol consumption, and other variables. Furthermore, it included a range of physical and laboratory examination parameters, such as body mass index (BMI), WC, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), fasting insulin (FINS), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCR), and serum uric acid (SUA).
Definitions
eGDR (mg/kg/min) was calculated using the following formula [21]:
WC: waist circumference (cm); HT: hypertension (yes = 1/no = 0); HbA1c: HbA1c (%)
According to the guidelines of the World Health Organization on diabetes [22], the diagnostic criteria for DM consist of FBG ≥ 7.0 mmol/L, 2-h plasma glucose level ≥ 11.1 mmol/L as per the oral glucose tolerance test, HbA1c ≥ 6.5%, or a history of T2DM. normal glucose regulation (NGR) is defined as FBG < 5.6 mmol/L or HbA1c < 5.7%. Pre-DM should be considered for individuals with elevated plasma glucose levels that do not meet the criteria for a T2DM diagnosis.
Ascertainment of mortality
In order to clarify the mortality outcomes of the follow-up population with CMS, we used the NHANES publicly available mortality files as of December 31, 2019. These files are matched by the National Center for Health Statistics (NCHS) using a probability matching algorithm with the National Death Index (NDI). The causes of death are examined using International Classification of Diseases 10th revision (ICD-10). The study endpoints included all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality. All-cause mortality encompassed deaths from any cause, including heart diseases, malignant neoplasms, and other causes. Cardiovascular specific mortality was defined by deaths due to heart diseases (ICD-10 codes I00–I09, I11, I13, I20–I51) and cerebrovascular diseases (ICD-10 codes I60–I69). Diabetes specific mortality referred to deaths caused by diabetes mellitus (ICD-10 codes E10–E14). Death data and details can be accessed through the website (https://www.cdc.gov/nchs/data-linkage/mortality.htm).
Statistical analysis
In this research analysis, weighting was conducted according to the requirements of NHANES, especially in the case of four-category variables where the weighted Q4 classification was used for analysis. In the baseline analysis, continuous variables were represented by median and interquartile range, while categorical data were expressed in percentages. Kruskal–Wallis test was applied for continuous variables and Chi-Squared test for categorical variables. Univariate and multivariate Cox proportional hazards models were used to estimate the relationship between eGDR levels and both all-cause and cause-specific mortality in CMS population with different glucose metabolism statuses. The analysis provided 95% confidence intervals (CIs) and hazard ratios (HRs) through three progressively adjusted models. Model 1 was the unadjusted analysis, while Model 2 included simple adjustments for age and gender. In the fully adjusted Model 3, in addition to considering the variables in Model 2, further adjustments were made for race, education, poverty, marital status, smoking, drinking, BMI, ALT, AST, TC, TG, SCR, SUA, and FINS. Additionally, Receiver Operating Characteristic (ROC) curves were constructed to predict all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality in the entire CMS population and across various glucose metabolic states based on eGDR and other IR-related indicators, with the corresponding areas under the curve (AUC) calculated. The restricted cubic spline (RCS) method (with 4 knots) was used to explore potential nonlinear relationships between eGDR levels (after adjustment in Model 3) and mortality rates. Subsequently, stratified analyses were conducted on CMS population adjusted by Model 3 to investigate situations within different subgroups and ascertain the presence of any interactions. A two-sided p < 0.05 was considered to be statistically significant. All analyses were performed with R version 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
Results
Weighted baseline characteristics of participants according to the eGDR quartiles
Table 1 showed the characteristics of participants (n = 9928) at baseline stratified by quartiles of eGDR levels. The average age of the participants was 55 years old, with a male proportion of 48.08%. In terms of glucose metabolism, the proportion of baseline DM and Pre-DM was relatively high, especially with baseline Pre-DM accounting for as high as 54.12%. According to the quartiles of eGDR levels, participants with higher eGDR levels were younger, had a higher proportion of non-smokers, and a lower proportion of BMI ≥ 30 (43.49%) participants compared to participants in the lowest quartile. Furthermore, there were significant differences between the two groups in terms of biochemical indicators. Compared to participants in the lowest quartile, those in the highest quartile showed lower levels of FBG, HBA1c, SCR, and SUA. Regarding WC, compared to participants in the lowest quartile, the WC levels in the Q3 and Q4 groups were lower, especially in the Q3 group. In terms of lipid profiles, participants in the highest quartile showed higher levels of TC, TG, LDL-C, and lower levels of HDL-C compared to participants in the lowest quartile. Additionally, in terms of mortality events, participants in the highest quartile had lower all-cause mortality (8.13%), cardiovascular specific mortality (1.80%), and diabetes specific mortality (0.71%) compared to participants in the lowest quartile (all p < 0.05).
Table 1.
Weighted baseline characteristics of participants
| Characteristics | Total N = 9928 (100%) |
Q1 N = 2580 (25%) |
Q2 N = 2589 (25%) |
Q3 N = 2494 (25%) |
Q4 N = 2265 (25%) |
p |
|---|---|---|---|---|---|---|
| Age | 55 (43, 66) | 57 (47, 67) | 59 (47, 69) | 56 (44, 68) | 47 (36, 58) | < 0.001 |
| Gender (n, %) | < 0.001 | |||||
| Male | 48.08 | 54.68 | 52.38 | 39.35 | 45.90 | |
| Female | 51.92 | 45.32 | 47.62 | 60.65 | 54.10 | |
| Race (n, %) | < 0.001 | |||||
| Mexican American | 7.85 | 5.94 | 6.32 | 7.35 | 11.78 | |
| Non-Hispanic black | 10.05 | 14.22 | 10.89 | 8.91 | 6.18 | |
| Non-Hispanic white | 70.80 | 71.50 | 72.74 | 70.71 | 68.23 | |
| Other | 11.31 | 8.34 | 10.05 | 13.03 | 13.80 | |
| Education (n, %) | 0.417 | |||||
| Below high school | 19.65 | 20.27 | 19.60 | 18.36 | 20.39 | |
| High School or above | 80.35 | 79.73 | 80.40 | 81.64 | 79.61 | |
| Married (n, %) | 65.57 | 63.17 | 67.23 | 64.96 | 66.93 | 0.101 |
| Poverty (n, %) | 19.93 | 20.42 | 19.18 | 20.06 | 20.07 | 0.791 |
| Drinking (n, %) | 61.45 | 59.45 | 63.76 | 58.69 | 63.89 | 0.018 |
| Smoking status (n, %) | < 0.001 | |||||
| Current Smoker | 19.77 | 16.10 | 16.93 | 22.50 | 23.54 | |
| Former Smoker | 30.59 | 37.52 | 34.56 | 25.93 | 24.37 | |
| Never Smoker | 49.64 | 46.37 | 48.51 | 51.57 | 52.10 | |
| BMI, kg/m2 (n, %) | < 0.001 | |||||
| < 25 | 9.57 | 0.59 | 3.53 | 21.83 | 12.33 | |
| 25–30 | 32.30 | 7.27 | 39.44 | 38.31 | 44.18 | |
| ≥ 30 | 58.12 | 92.14 | 57.03 | 39.87 | 43.49 | |
| Glucose metabolic states | < 0.001 | |||||
| DM (n, %) | 25.74 | 51.26 | 23.34 | 17.72 | 10.64 | |
| Pre-DM (n, %) | 54.12 | 38.54 | 56.02 | 58.82 | 63.10 | |
| NGR (n, %) | 20.14 | 10.20 | 20.64 | 23.46 | 26.26 | |
| FBG, mmol/L | 5.91 (5.55, 6.55) | 6.50 (5.77, 8.16) | 5.89 (5.50, 6.49) | 5.83 (5.44, 6.27) | 5.77 (5.44, 6.11) | < 0.001 |
| HBA1c, % | 5.60 (5.30, 6.00) | 6.00 (5.60, 7.10) | 5.60 (5.40, 6.00) | 5.50 (5.30, 5.90) | 5.50 (5.20, 5.70) | < 0.001 |
| FINS, uU/mL | 13.40 (8.92, 20.59) | 18.93 (12.32, 29.47) | 12.94 (8.94, 18.51) | 11.49 (7.55, 17.77) | 12.28 (8.32, 17.62) | < 0.001 |
| TC, mmol/L | 5.04 (4.37, 5.82) | 4.76 (4.14, 5.48) | 5.04 (4.37, 5.84) | 5.20 (4.45, 5.95) | 5.20 (4.53, 5.97) | < 0.001 |
| TG, mmol/L | 1.63 (1.13, 2.21) | 1.58 (1.14, 2.19) | 1.54 (1.08, 2.13) | 1.54 (1.05, 2.17) | 1.80 (1.24, 2.34) | < 0.001 |
| HDL-C, mmol/L | 1.16 (1.00, 1.40) | 1.16 (0.98, 1.34) | 1.22 (1.03, 1.47) | 1.24 (1.01, 1.55) | 1.09 (0.96, 1.29) | < 0.001 |
| LDL-C, mmol/L | 3.03 (2.41, 3.67) | 2.77 (2.20, 3.47) | 2.97 (2.38, 3.67) | 3.08 (2.46, 3.72) | 3.21 (2.64, 3.80) | < 0.001 |
| ALT, IU/L | 23 (17, 31) | 24 (18, 33) | 23(18, 31) | 22.00 (17, 30) | 23.00 (17, 31) | < 0.001 |
| AST, IU/L | 23 (19, 28) | 23.00 (19, 29) | 23.00 (19, 28) | 23.00 (19, 27) | 22.00 (19, 27) | < 0.001 |
| SCR, umol/L | 75.14 (62.76, 88.40) | 78.68 (65.42, 90.17) | 77.79 (65.42, 91.05) | 73.37 (61.88, 88.40) | 70.72 (61.88, 84.86) | < 0.001 |
| SUA, mg/dL | 5.80 (4.90, 6.80) | 6.20 (5.30, 7.20) | 5.90 (5.00, 6.90) | 5.60 (4.70, 6.50) | 5.50 (4.60, 6.30) | < 0.001 |
| WC, cm | 106.80 (98.20, 116.70) | 121.50 (115.50, 130.10) | 106.40 (102.90, 110.20) | 97.00 (92.00, 116.00) | 102.20 (94.70, 107.00) | < 0.001 |
| eGDR, mg/kg/min | 5.70 (4.40, 7.80) | 3.37 (2.47, 3.98) | 5.08 (4.76, 5.39) | 6.48 (6.06, 7.05) | 8.90 (8.42, 9.53) | < 0.001 |
| Mortality (%) | ||||||
| All-cause | 14.98 | 18.13 | 17.29 | 16.40 | 8.13 | < 0.001 |
| Cardiovascular | 4.69 | 6.72 | 5.63 | 4.62 | 1.80 | < 0.001 |
| Diabetes | 2.05 | 4.37 | 1.75 | 1.37 | 0.71 | < 0.001 |
BMI: body mass index; DM: diabetes mellitus; Pre-DM: pre-diabetes mellitus; NGR: normal glucose regulation; FBG: fasting blood glucose; HBA1c: Hemoglobin A1c; FINS: fasting insulin; TC: total cholesterol; TG: triglycerides; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; ALT: alanine aminotransferase; AST: aspartate aminotransferase; SCR: serum creatinine; SUA: serum uric acid; WC: waist circumference; eGDR: estimated glucose disposal rate
Weighted baseline characteristics of participants according to survival status
When stratifying CMS participants based on their survival status, the study results showed that deceased CMS patients generally exhibited lower eGDR levels, while TG, HBA1c, and FBG levels were relatively higher, showing significant differences compared to the surviving group. Concerning glucose metabolic state, the prevalence of DM among the deceased group was 38.37%, whereas it was 23.51% in the survival group. The combined prevalence of baseline DM and Pre-DM in the deceased group was notably higher at 86.92%, as opposed to 78.61% in the surviving population (all p < 0.05). Detailed results were available in Additional file 1 (Table S1).
Association between eGDR levels with mortality of CMS participants
In Table 2, Cox regression analysis showed that whether eGDR levels were considered as continuous variables or categorical variables, eGDR levels were significantly associated with all-cause mortality and cause-specific mortality in the CMS population. When eGDR levels were considered as continuous variables, the fully adjusted model showed that the HR value for eGDR levels associated with all-cause mortality in the CMS population was 0.90 (95% CI 0.87–0.93, p < 0.001), for cardiovascular specific mortality was 0.84 (95% CI 0.79–0.89, p < 0.001), and for diabetes specific mortality was 0.71 (95% CI 0.64–0.78, p < 0.001). When eGDR levels were considered as categorical variables, compared to the first quartile of eGDR levels, the HR value for the fourth quartile of eGDR levels was 0.57 (95% CI 0.46–0.70, p < 0.001) in terms of all-cause mortality, 0.38 (95% CI 0.26–0.56, p < 0.001) in terms of cardiovascular specific mortality, and 0.17 (95% CI 0.09–0.31, p < 0.001) in terms of diabetes specific mortality.
Table 2.
Association between eGDR levels with all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality of CMS participants
| Death (n) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | ||
| All-cause mortality | |||||||
| Continuous | 1936 | 0.86 (0.84–0.88) | < 0.001 | 0.91 (0.88–0.94) | < 0.001 | 0.90 (0.87–0.93) | < 0.001 |
| Q1 | 598 | Ref | Ref | Ref | |||
| Q2 | 585 | 0.83 (0.72–0.96) | 0.012 | 0.75 (0.65–0.86) | < 0.001 | 0.78 (0.67–0.91) | 0.002 |
| Q3 | 522 | 0.78 (0.66–0.93) | 0.005 | 0.80 (0.67–0.96) | 0.016 | 0.78 (0.63–0.96) | 0.016 |
| Q4 | 231 | 0.33 (0.27–0.40) | < 0.001 | 0.57 (0.47–0.70) | < 0.001 | 0.57 (0.46–0.70) | < 0.001 |
| p-trend | < 0.001 | < 0.001 | < 0.001 | ||||
| Cardiovascular specific mortality | |||||||
| Continuous | 637 | 0.81 (0.78–0.84) | < 0.001 | 0.84 (0.79–0.89) | < 0.001 | 0.84 (0.79–0.89) | < 0.001 |
| Q1 | 217 | Ref | Ref | Ref | |||
| Q2 | 206 | 0.73 (0.59–0.91) | 0.005 | 0.64 (0.51–0.80) | < 0.001 | 0.73 (0.55–0.97) | 0.028 |
| Q3 | 160 | 0.59 (0.45–0.77) | < 0.001 | 0.60 (0.46–0.78) | < 0.001 | 0.63 (0.45–0.88) | 0.007 |
| Q4 | 54 | 0.20 (0.14–0.28) | < 0.001 | 0.36 (0.25–0.52) | < 0.001 | 0.38 (0.26–0.56) | < 0.001 |
| p-trend | < 0.001 | < 0.001 | < 0.001 | ||||
| Diabetes specific mortality | |||||||
| Continuous | 290 | 0.71 (0.66–0.76) | < 0.001 | 0.70 (0.64–0.77) | < 0.001 | 0.71 (0.64–0.78) | < 0.001 |
| Q1 | 141 | Ref | Ref | Ref | |||
| Q2 | 71 | 0.35 (0.25–0.49) | < 0.001 | 0.32 (0.23–0.46) | < 0.001 | 0.32 (0.21–0.49) | < 0.001 |
| Q3 | 53 | 0.27 (0.18–0.40) | < 0.001 | 0.28 (0.19–0.41) | < 0.001 | 0.23 (0.14–0.39) | < 0.001 |
| Q4 | 25 | 0.12 (0.07–0.21) | < 0.001 | 0.19 (0.11–0.34) | < 0.001 | 0.17 (0.09–0.31) | < 0.001 |
| p-trend | < 0.001 | < 0.001 | < 0.001 | ||||
eGDR: Estimated glucose disposal rate; CMS: Cardiometabolic syndrome; HR: Hazard ratios; CI: Confidence interval
Non-linear trends of eGDR levels with mortality of CMS participants
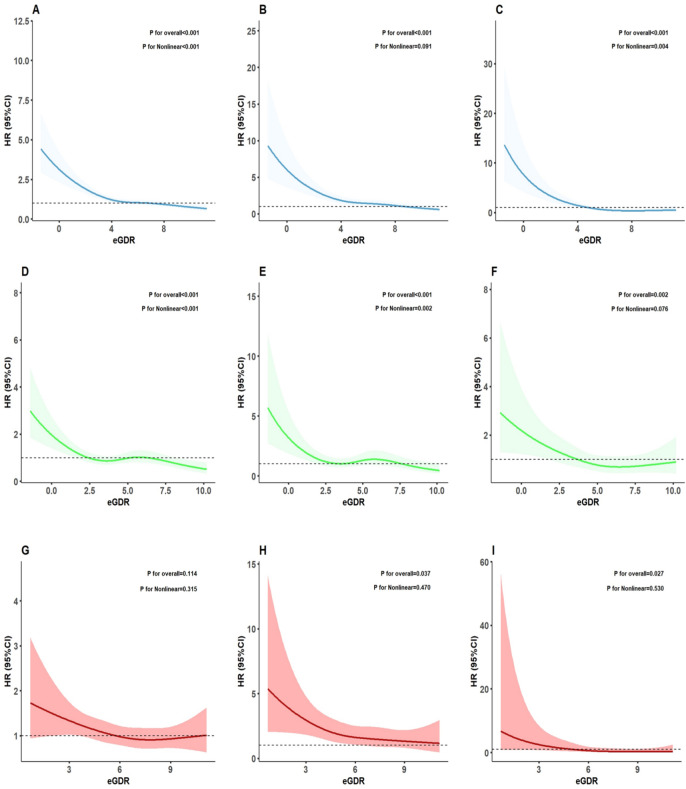
As shown in Fig. 2, we conducted RCS analysis to visualize the relationship between eGDR levels and all-cause mortality and cause-specific mortality in the CMS participants. In the CMS participants, eGDR levels showed a non-linear relationship with all-cause mortality (p for overall < 0.001, p for non-linear < 0.001, Fig. 2A) and diabetes specific mortality (p for overall < 0.001, p for non-linear = 0.004, Fig. 2C), while it had a linear relationship with cardiovascular specific mortality (p for overall < 0.001, p for non-linear = 0.091, Fig. 2B). Segmental Cox regression identified eGDR thresholds for all-cause and diabetes specific mortality in the entire CMS population as 8.22 and 5.61, respectively (Table S2).
Fig. 2.
RCS of the relationship between eGDR levels and the occurrence of all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality among CMS participants with different glucose metabolism statuses. A–C represents the relationship between eGDR and all-cause mortality (A), cardiovascular specific mortality (B), and diabetes specific mortality (C) in the entire CMS population. D–F represents the relationship between eGDR and all-cause mortality (D), cardiovascular specific mortality (E), and diabetes specific mortality (F) in the CMS population with baseline diabetes. G, I represents the relationship between eGDR and all-cause mortality (G), cardiovascular specific mortality (H), and diabetes specific mortality (I) in the CMS population with baseline pre-diabetes. RCS: restricted cubic spline; HR: hazard ratios; CI: confidence interval; eGDR: estimated glucose disposal rate; CMS: cardiometabolic syndrome
Stratified analyses of the associations between eGDR levels and mortality
As shown in Fig. 3, there was a strong correlation between eGDR levels and all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality in various subgroups of participants with CMS in terms of different age, gender, race, education, BMI, and smoking (all p < 0.05). Furthermore, subgroup analysis revealed some significant interaction effects (all p < 0.05), indicating significant differences in the relationship between eGDR levels and both all-cause mortality (Fig. 3A) and cardiovascular specific mortality (Fig. 3B) in different subgroups of participants with CMS, such as in terms of age and race. Specifically, the protective effect of eGDR levels was most pronounced in participants under the age of 50, resulting in a 26% decrease in all-cause mortality (Fig. 3A) and a 31% decrease in cardiovascular specific mortality (Fig. 3B). Furthermore, the impact of eGDR levels on mortality in the CMS population varies across subgroups defined by age, race, and smoking status under different glucose metabolism states (Figures S1-S6).
Fig. 3.
Stratified analyses of the associations between eGDR levels and the occurrence of all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality in CMS participants.Youth: age < 50 years; Middle-aged: 50 years ≤ age ≤ 64 years; Elderly: age > 64 years. eGDR: estimated glucose disposal rate; CMS: cardiometabolic syndrome; HR: hazard ratios; CI: confidence interval; BMI: body mass index
Association between eGDR levels and mortality in CMS participants with different glucose metabolic states
As shown in Table 3, In baseline DM participants, eGDR levels were significantly associated with all-cause, cardiovascular specific mortality, and diabetes specific mortality. When eGDR was considered as a continuous variable, the respective HR were 0.92 (95%CI 0.87–0.97, p = 0.004), 0.89 (95%CI 0.81–0.98, p = 0.016), and 0.89 (95%CI 0.81–0.97, p = 0.012). When considered as a categorical variable, compared to Q1, the respective HR for Q4 were 0.73 (95%CI (0.53–1.00, p = 0.048), 0.63 (95%CI 0.37–1.08, p = 0.095) and 0.47 (95%CI 0.28–0.79, p = 0.005). In the Pre-DM participants, eGDR levels were significantly associated with cardiovascular and diabetes specific mortality. When eGDR was considered as a continuous variable, the respective HR were 0.89 (95%CI 0.80–0.98, p = 0.021) and 0.71 (95%CI 0.55–0.91, p = 0.008). When considered as a categorical variable, compared to Q1 group, the respective HR for Q4 group were 0.52 (95%CI 0.28–0.96, p = 0.037) and 0.10 (95%CI 0.03–0.34, p < 0.001), but no significance was observed in all-cause mortality. In the baseline NGR participants, eGDR levels showed no significant association with all-cause and cardiovascular specific mortality, but were significantly associated with diabetes specific mortality (HR = 0.21, 95%CI 0.08–0.60, p = 0.003). The corrected results of various models were shown in Tables S3-5.
Table 3.
Association between eGDR levels with all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality of CMS participants in different glucose metabolic states at baseline
| All-cause mortality (Model 3) |
Cardiovascular specific mortality (Model 3) |
Diabetes specific mortality (Model 3) |
||||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Baseline DM | ||||||
| Continuous | 0.92 (0.87–0.97) | 0.004 | 0.89 (0.81–0.98) | 0.016 | 0.89 (0.81–0.97) | 0.012 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.63 (0.47–0.84) | 0.002 | 0.58 (0.35–0.96) | 0.033 | 0.46 (0.29–0.73) | 0.001 |
| Q3 | 0.73 (0.55–0.95) | 0.025 | 0.80 (0.50–1.30) | 0.373 | 0.39 (0.23–0.67) | < 0.001 |
| Q4 | 0.73 (0.53–1.00) | 0.048 | 0.63 (0.37–1.08) | 0.095 | 0.47 (0.28–0.79) | 0.005 |
| p-trend | 0.124 | 0.237 | 0.008 | |||
| Baseline Pre-DM | ||||||
| Continuous | 0.95 (0.90–1.01) | 0.099 | 0.89 (0.80–0.98) | 0.021 | 0.71 (0.55–0.91) | 0.008 |
| Q1 | Ref | Ref | Ref | |||
| Q2 | 0.85 (0.66–1.08) | 0.172 | 0.58 (0.38–0.88) | 0.011 | 0.21 (0.07–0.65) | 0.007 |
| Q3 | 0.75 (0.56–1.01) | 0.059 | 0.55 (0.34–0.88) | 0.014 | 0.19 (0.07–0.54) | 0.002 |
| Q4 | 0.79 (0.56–1.10) | 0.159 | 0.52 (0.28–0.96) | 0.037 | 0.10 (0.03–0.34) | < 0.001 |
| p-trend | 0.119 | 0.040 | < 0.001 | |||
| Baseline NGR | ||||||
| Continuous | 0.88 (0.76–1.02) | 0.083 | 0.80 (0.63–1.03) | 0.080 | 0.21 (0.08–0.60) | 0.003 |
eGDR: Estimated glucose disposal rate; CMS: Cardiometabolic syndrome; HR: Hazard ratios; CI: Confidence interval; DM: Diabetes mellitus; Pre-DM: Pre-diabetes mellitus; NGR: Normal glucose regulation
As shown in Fig. 2, The RCS analysis results indicated that in the CMS participants with baseline DM, the eGDR levels exhibited a non-linear relationship with all-cause mortality (p for overall < 0.001, p for non-linear < 0.001, Fig. 2D) as well as cardiovascular specific mortality (p for overall < 0.001, p for non-linear = 0.002, Fig. 2E). However, the relationship with diabetes specific mortality showed a linear correlation (p for overall = 0.002, p for non-linear = 0.076, Fig. 2F). Segmental Cox regression identified eGDR thresholds for all-cause and cardiovascular specific mortality in the CMS participants with baseline DM as 4.94 and 3.08, respectively (Table S2). In the baseline Pre-DM participants, eGDR levels showed a linear relationship with cardiovascular specific mortality (p for overall = 0.037, p for non-linear = 0.470, Fig. 2H) and diabetes specific mortality (p for overall = 0.027, p for non-linear = 0.530, Fig. 2I).
ROC curve analysis of IR-related indicators for predicting mortality in the entire CMS population and across various glucose metabolic states
To thoroughly assess the impact of glucose metabolic status on the predictive value of eGDR for all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality, as well as to evaluate the differences in predictive performance among various IR-related indicators, ROC curves were plotted, and the AUC along with the corresponding 95% CIs, cut off, sensitivity, and specificity were calculated (Fig. 4, Table S6). The results indicated that in the entire CMS population and among those with Pre-DM at baseline, eGDR demonstrated superior predictive value for all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality compared to TyG and HOMA-IR. Moreover, compared with the DM, IR-related indicators showed stronger predictive value for mortality in both Pre-DM and NGR populations. However, it is important to note that the AUC for IR-related indicators was below 0.7 across all glucose states.
Fig. 4.
ROC curve analysis of IR-related indicators for predicting mortality in the entire CMS population and across various glucose metabolic states. A–C Represents the predictive values of IR-related indicators for all-cause mortality (A), cardiovascular specific mortality (B), and diabetes specific mortality (C) in the entire CMS population. D–F represents the predictive values of IR-related indicators for all-cause mortality (D), cardiovascular specific mortality (E), and diabetes specific mortality (F) in the CMS population with baseline diabetes. G, I represents the predictive values of IR-related indicators for all-cause mortality (G), cardiovascular specific mortality (H), and diabetes specific mortality (I) in the CMS population with baseline pre-diabetes. J–L represents the predictive values of IR-related indicators for all-cause mortality (J), cardiovascular specific mortality (K), and diabetes specific mortality (L) in the CMS population with baseline normal glucose regulation. ROC: receiver operating characteristic; CMS: cardiometabolic syndrome; IR: insulin resistance; CI: confidence interval; eGDR: estimated glucose disposal rate; HOMA-IR: homeostasis model assessment of insulin resistance; TyG: triglyceride glucose
As shown in Table S6, in terms of accuracy and sensitivity, eGDR demonstrates outstanding performance across all clinical outcomes (all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality), particularly showing high sensitivity in all-cause mortality and cardiovascular specific mortality. TyG exhibits a balanced performance in both sensitivity and accuracy, especially excelling in diabetes specific mortality, whereas HOMA-IR generally shows lower sensitivity and accuracy, with its overall performance lagging behind that of eGDR and TyG.
Discussion
To our knowledge, this study explored for the first time the relationship between eGDR levels and the all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality in population with CMS in different glucose metabolic states. Our study found that high levels of eGDR were significantly negatively correlated with the risks of all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality in the CMS participants. Furthermore, the RCS analysis showed that there was a non-linear relationship between eGDR levels and all-cause mortality as well as diabetes specific mortality in CMS participants, while the relationship with cardiovascular specific mortality was linear. Moreover, the inverse association between eGDR and mortality persists across various glucose metabolic states.
CMS is a syndrome characterized by a series of metabolic disorders, with factors such as IR, lipid disorders, hypertension, and central obesity playing crucial roles in its occurrence and development [3, 4]. It significantly increases the risk of cardiovascular diseases and T2DM in the CMS population, leading to a significant increase in related mortality events [23–25]. As a marker of IR, eGDR integrates multiple metabolic risk factors. Research has confirmed the value of eGDR in prognosis in diabetic populations [26–30]. Ren et al. found that in a 6-year follow-up study of the general population in China, higher levels of eGDR were associated with reduced risk of cardiovascular disease and stroke events [19]. Furthermore, eGDR levels also exhibit a protective effect in non-diabetic patients with chronic kidney disease. Peng et al. found that higher levels of eGDR not only lower the incidence of cardiovascular diseases such as coronary artery disease and stroke, but also reduce all-cause and cause-specific mortality. This result was validated in the UK Biobank and NHANES cohorts [31]. However, the prognostic significance of eGDR in disorders like CMS, which are caused by multiple metabolic abnormalities, is still uncertain. Our study found a significant association between eGDR levels and the prognosis of CMS populations, where lower eGDR levels significantly increased all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality. Additionally, RCS analysis revealed a non-linear relationship between eGDR levels and all-cause mortality and diabetes specific mortality in the CMS population, while a linear relationship was observed with cardiovascular specific mortality. This finding is consistent with previous research results. For instance, the study by Zhang et al. demonstrated a linear association between eGDR level and the incidence of cardiovascular disease [17]. Furthermore, the NHANES study by He et al. also further confirmed the linear relationship between eGDR level and cardiovascular-related mortality [32]. Additionally, our study found specific thresholds for eGDR in relation to all-cause mortality and diabetes specific mortality in entire CMS population, which are 8.22 and 5.61, respectively. This finding suggests that when eGDR levels fall below these thresholds, an increase in eGDR levels may have a more pronounced positive impact on reducing mortality.
A study on the entire US population found that high eGDR levels reduced all-cause mortality regardless of age < 60 or ≥ 60 years [33]. Our study results were similar, and subgroup analysis showed a significant interaction between eGDR levels and all-cause mortality and cardiovascular specific mortality in different age subgroups of the CMS population. High eGDR levels have varying protective effects on different age groups within the CMS population, particularly demonstrating a significant impact on those under 50 years old, reducing all-cause mortality by 26% and cardiovascular specific mortality by 31%.
CMS populations commonly have abnormalities in glucose metabolism. This study found that in CMS populations, diabetes and prediabetes accounted for 25.74% and 54.12%, respectively. Therefore, investigating the prognosis of CMS populations under different glucose metabolic states is particularly important. Our ROC results indicate that, under different glucose metabolic states, compared with DM patients, eGDR has a higher predictive value for all-cause mortality, cardiovascular specific mortality, and diabetes specific mortality in individuals with CMS who have Pre-DM or NGR at baseline. This finding is similar to the results of He et al. [34]. Moreover, Kong et al.'s NHANES study found that in Pre-DM populations, lower eGDR levels were associated with increased all-cause mortality and cardiovascular specific mortality [35]. Additionally, low eGDR levels significantly increase mortality in both type 1 and type 2 diabetes, as indicated in other studies [16, 26, 36]. Our study showed that regardless of the glucose metabolism status, a decrease in eGDR levels was associated with an increase in diabetes specific mortality in the CMS population. Additionally, low eGDR levels were associated with an increased cardiovascular specific mortality in individuals with DM and Pre-DM in the CMS population. At the same time, low eGDR levels were closely related to all-cause mortality in the CMS population with DM. He et al. found in a NHANES study of the entire US population that a high eGDR provides stronger protection against cardiovascular and all-cause mortality risks for individuals with NGR and Pre-DM compared to those with DM [34]. This difference may be due to the varying characteristics of the populations. In the CMS population with NGR, it often implies more severe metabolic disorders, especially lipid abnormalities, central obesity, and hypertension, which are key risk factors for all-cause and cardiovascular mortality. Therefore, the effect of IR-related indicators may be reduced in this process [37–39]. Our ROC curve analysis results also confirmed this point. Compared with all-cause and cardiovascular specific mortality, the eGDR level has a stronger risk prediction for diabetes specific mortality. These results underscore the importance of evaluating individual prognosis based on eGDR levels in the CMS population with glucose metabolism abnormalities.
Although the underlying mechanisms of the eGDR levels in the occurrence and prognosis of CMS have not yet been fully elucidated, increasing evidence suggests its close association with IR. The potential pathophysiological pathways may involve multiple interrelated mechanisms. IR may impair myocardial energy metabolism efficiency by reducing glucose utilization and altering substrate preferences [40, 41]. Simultaneously, IR can promote oxidative stress and disrupt lipid metabolism, leading to vascular endothelial dysfunction and atherosclerosis, among other effects [42–44]. The synergistic effects of these mechanisms may significantly increase the risk of morbidity and mortality from CMS.
Strengths and limitations
The strength of this study lies in it being the first to investigate the relationship between eGDR levels and all-cause mortality and cause-specific mortality in the CMS population. Additionally, it delves further into exploring the relationship between eGDR levels and mortality under different glucose metabolism states. However, this study also has some limitations. Firstly, this cohort study is limited to data from a single country, thus requiring further validation in other populations. Secondly, the calculation of eGDR levels in this study is based solely on the baseline results of the study population, lacking monitoring of its dynamic changes and therefore unable to determine the long-term status of eGDR levels in the study population. Moreover, in the process of calculating eGDR, information on whether the study participants have hypertension is needed. Despite efforts to include self-reported hypertension, current use of antihypertensive medications, and the average of three consecutive blood pressure measurements as criteria for hypertension diagnosis, there may still be unidentified individuals with hypertension, potentially impacting the study results. Given the retrospective nature of the study, residual confounding factors may persist, despite efforts to control for them, such as family history and medication history. Additionally, in the analysis of different glucose metabolism states, due to the low number of deaths in the NGR population, eGDR cannot be considered as a categorical variable for further analysis, warranting a larger study in the future.
Conclusion
In the entire CMS population, there is a significant correlation between eGDR levels and both all-cause and cause-specific mortality. Furthermore, the protective effect of high eGDR levels on mortality persists across various glucose metabolic states.
Supplementary Information
Acknowledgements
We want to acknowledge the participants and investigators of the NHANES and NDI database.
Author contributions
The individual contributions of the authors are as follows: Conception and design: CF, YL, and QZ; Collection and assembly of data: GW, XM, XG, SL, YG, and BH; Statistical analysis: HW, HZ, FW and BH; Manuscript drafting: CF and YL; Revisions of the manuscript: CF, GW, XM, XG. All authors reviewed the manuscript.
Funding
This study was supported by the National Clinical Research Center for Geriatric Diseases (NCRCG-PLAGH-2024002) and Noncommunicable Chronic Diseases-National Science and Technology Major Project (No. 2023ZD0508300).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All survey protocols were approved by the institutional review board of the National Center for Health Statistics.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chao Fu and Yuxin Li have contributed equally to this work.
Contributor Information
Fei Wang, Email: wangfei301hmi@126.com.
Qiang Zeng, Email: ZQ301@126.com.
References
- 1.Mohammad GS. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maria A, Taft B, Sharon T, Benny L, Robert JW. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–4. 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 3.George MMAK, Paul Z, Jonathan S. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–62. 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 4.Naveed S, Jason MRG, William A. Improving prevention strategies for cardiometabolic disease. Nat Med. 2020;26(3):320–5. 10.1038/s41591-020-0786-7. [DOI] [PubMed] [Google Scholar]
- 5.Zoran G, Bozidarka Z, Ivana R, Milan O, Aleksandar M, Djordje R, Esma RI. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol. 2016;15(1):30–9. 10.2174/1570161114666161007164510. [DOI] [PubMed] [Google Scholar]
- 6.Jean-Pierre D, Isabelle L. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 7.Mike M. Metabolic syndrome recasts old cardiac, diabetes risk factors as a “new” entity. JAMA. 2004;291(17):2062–3. 10.1001/jama.291.17.2062. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 9.Charmaine ST, Wenting X, William DJ, William TC, Leanne MR, Eric R. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–10. 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dicky Levenus T, Livy Bonita P, Nissha Audina F, Cicilia M, Syahidatul W, Farid K, Aulia R, Tri Juli Edi T, Dante Saksono H, Dyah P, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581. 10.1016/j.dsx.2022.102581. [DOI] [PubMed] [Google Scholar]
- 11.Xiao L, Ziqi T, Yuna H, Huilei Z, Menglu L, Peng Y, Jianyong M, Yujie Z, Wengen Z, Jingfeng W. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124. 10.1186/s12933-022-01546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rui S, Jianxin W, Meng L, Jingen L, Yi P, Birong L, Gregory YHL, Lijing Z. Association of insulin resistance with cardiovascular disease and all-cause mortality in type 1 diabetes: systematic review and meta-analysis. Diabetes Care. 2024;47(12):2266–74. 10.2337/dc24-0475. [DOI] [PubMed] [Google Scholar]
- 13.Wedén L, Martina P, Björn R, Johnny L, Marcus L, Mikael AF, Thomas N. Estimated glucose disposal rate is associated with retinopathy and kidney disease in young people with type 1 diabetes: a nationwide observational study. Cardiovasc Diabetol. 2023;22(1):61. 10.1186/s12933-023-01791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuanpin Z, Wanwan S, Qi Z, Yuetian B, Lijin J, Hangping Z, Xiaoming Z, Xiaoxia L, Shuo Z, Qian X, et al. Estimated glucose disposal rate predicts the risk of diabetic peripheral neuropathy in type 2 diabetes: a 5-year follow-up study. J Diabetes. 2024;16(5): e13482. 10.1111/1753-0407.13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juan P, Aimei L, Liangqingqing Y, Qi Y, Jinting P, Bin Y. Estimated glucose disposal rate predicts renal progression in type 2 diabetes mellitus: a retrospective cohort study. J Endocr Soc. 2023;7(7): bvad069. 10.1210/jendso/bvad069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuseppe P, Anna S, Emanuela O, Enzo B, Cecilia F, Roberto T, Monica V, Franco C, Gianpaolo Z, Olga L, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021;19(1):66. 10.1186/s12916-021-01936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zenglei Z, Lin Z, Yiting L, Yan X, Xianliang Z. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: findings from a nationwide, population based, prospective cohort study. Cardiovasc Diabetol. 2024;23(1):194. 10.1186/s12933-024-02256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhengzhao L, Yunyun X, Xueyan F, Kaixuan Y, Hongqiu G, Xingquan Z, Xia M, Yongjun W. Insulin resistance estimated by estimated glucose disposal rate predicts outcomes in acute ischemic stroke patients. Cardiovasc Diabetol. 2023;22(1):225. 10.1186/s12933-023-01925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao R, Minglan J, Longyang H, Xiaowei Z. Estimated glucose disposal rate and risk of cardiovascular disease: evidence from the China Health and Retirement Longitudinal Study. BMC Geriatr. 2022;22(1):968. 10.1186/s12877-022-03689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleeman JI. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–97. 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Nyström T, Holzmann M, Eliasson B, Svensson A, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–63. 10.1111/dom.13110. [DOI] [PubMed] [Google Scholar]
- 22.Rydén L, Grant P, Anker S, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes H, Huikuri H, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD—summary. Diab Vasc Dis Res. 2014;11(3):133–73. 10.1177/1479164114525548. [DOI] [PubMed] [Google Scholar]
- 23.Muhammad Imtiaz A, Michael DS. Preventing diabetes and atherosclerosis in the cardiometabolic syndrome. Curr Atheroscler Rep. 2021;23(4):16. 10.1007/s11883-021-00913-8. [DOI] [PubMed] [Google Scholar]
- 24.Antonio G-C, José Alejandro C-F, Sandra E-A, Alonso G-T, José Israel L-P, Cesar O. Metabolic syndrome and cardiovascular disease: a health challenge. Arch Med Res. 2018;49(8):516–21. 10.1016/j.arcmed.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Gerald R. Insulin resistance, type 2 diabetes mellitus, and cardiovascular disease: the end of the beginning. Circulation. 2005;112(20):3030–2. 10.1161/CIRCULATIONAHA.105.504670. [DOI] [PubMed] [Google Scholar]
- 26.Thomas N, Martin JH, Björn E, Ann-Marie S, Ulrik S. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2017;20(3):556–63. 10.1111/dom.13110. [DOI] [PubMed] [Google Scholar]
- 27.Rebecca H, Harriet W, Noppadol K, Matthew C, Rebecca B, Sam MP, Ramzi AA. Body mass index, estimated glucose disposal rate and vascular complications in type 1 diabetes: beyond glycated haemoglobin. Diabet Med. 2021;38(5): e14529. 10.1111/dme.14529. [DOI] [PubMed] [Google Scholar]
- 28.Juan JC, Alberto G, Juana AF-L-R, David B, María JC, Jaume P, Juan FC-P, Juan PB. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab. 2009;94(9):3530–4. 10.1210/jc.2009-0960. [DOI] [PubMed] [Google Scholar]
- 29.Christian MG, Benjamin DS, Kris EJP. Utility of the estimated glucose disposal rate as a marker of microvascular complications in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2012;96(3):e70–2. 10.1016/j.diabres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Monia G, Elisa G, Maria Giovanna S, Cristina B, Michele A, Fabrizio C, Daniela L, Giuseppe D, Roberto M, Paolo F, et al. Insulin resistance and risk of major vascular events and all-cause mortality in type 1 diabetes: a 10-year follow-up study. Diabetes Care. 2020;43(10):e139–41. 10.2337/dc20-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juan P, Yan Z, Yiqun Z, Weilin C, Li C, Fangyu M, Bin Y, Zhijun H. Estimated glucose disposal rate for predicting cardiovascular events and mortality in patients with non-diabetic chronic kidney disease: a prospective cohort study. BMC Med. 2024;22(1):411. 10.1186/s12916-024-03582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao-Ming H, Ying-Ying X, Qiang C, Yi-Ke L, Xue-Xi L, Ya-Kun M, Xiao-Yan D, Yan-Xiang G, Jin-Gang Z. The additive effect of the triglyceride-glucose index and estimated glucose disposal rate on long-term mortality among individuals with and without diabetes: a population-based study. Cardiovasc Diabetol. 2024;23(1):307. 10.1186/s12933-024-02396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubing G, Jingjing T, Yongtong C, Wei Z. Association between estimated glucose disposal rate and cardiovascular mortality across the spectrum of glucose tolerance in the US population. Diabetes Obes Metab. 2024;26(12):5827–35. 10.1111/dom.15954. [DOI] [PubMed] [Google Scholar]
- 34.He S, Wang C, Huang X, Jian G, Lu Z, Jiang K, Xie G, Sheng G, Zou Y. Analyzing the impact of glycemic metabolic status on cardiovascular mortality and all-cause mortality related to the estimated glucose disposal rate: a nationwide cohort study. Front Endocrinol. 2025;15:1494820. 10.3389/fendo.2024.1494820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiufang K, Wei W. Estimated glucose disposal rate and risk of cardiovascular disease and mortality in U.S. adults with prediabetes: a nationwide cross-sectional and prospective cohort study. Acta Diabetol. 2024;61(11):1413–21. 10.1007/s00592-024-02305-1. [DOI] [PubMed] [Google Scholar]
- 36.Alexander Z, Vladimer D, Marcus L, Ann-Marie S, Stefan F, Björn E, Cesare P, Magnus J, Thomas N. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2021;20(1):202. 10.1186/s12933-021-01394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramírez PC, de Oliveira Máximo R, de Oliveira DC, de Souza AF, Luiz MM, Delinocente MB, Steptoe A, de Oliveira C, da Silva Alexandre T. Dynapenic abdominal obesity as a risk factor for metabolic syndrome in individual 50 years of age or older: english longitudinal study of ageing. J Nutr Health Aging. 2023;27(12):1188–95. 10.1007/s12603-023-2039-1. [DOI] [PubMed] [Google Scholar]
- 38.Juan Antonio P. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. 2016;7(19):483–514. 10.4239/wjd.v7.i19.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen-Jung W, Tung-Wei K, Yuan-Yuei C, Hui-Fang Y, Wei-Liang C. Peripheral fat distribution versus waist circumference for predicting mortality in metabolic syndrome. Diabetes Metab Res Rev. 2018;35(4): e3116. 10.1002/dmrr.3116. [DOI] [PubMed] [Google Scholar]
- 40.Elena S, Francesco C, Annalisa P, Sofia M, Patrizia V, Teresa Vanessa F, Maria P, Angela S, Pietro Hiram G, Pierangelo V, et al. Impaired insulin-stimulated myocardial glucose metabolic rate is associated with reduced estimated myocardial energetic efficiency in subjects with different degrees of glucose tolerance. Cardiovasc Diabetol. 2023;22(1):4. 10.1186/s12933-022-01733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiara MAC, Alessia R, Teresa Vanessa F, Elena S, Maria P, Angela S, Francesco A, Giorgio S. Impaired insulin sensitivity measured by estimated glucose disposal rate is associated with decreased myocardial mechano-energetic efficiency in non-diabetic individuals. Eur J Intern Med. 2024;130:144–50. 10.1016/j.ejim.2024.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Antonino DP, Ralph AD. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. 10.1210/er.2018-00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert S, Massimo F. Insulin resistance and atherosclerosis: convergence between metabolic pathways and inflammatory nodes. Biochem J. 2013;454(1):1–11. 10.1042/BJ20130121. [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links: the Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–87. 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.