Abstract
Background
The natural compound cedrol possess anti-inflammatory and antioxidant properties. We sought to assess the neuroprotective effect of cedrol in an animal model of transient global ischemia/reperfusion (I/R) injury.
Method
To induce transient global cerebral I/R injury, bilateral carotid arteries were temporarily occluded for 20 min. A total of 40 male Wistar rats were randomly divided in to 5 groups; The control and global I/R groups, and the treatment groups that received cedrol at doses of 7.5, 15, and 30 mg/kg/day for a week, following the global I/R induction. The passive avoidance test was used for assessing memory function, and then hippocampal tissues were collected to assess levels of malondialdehyde (MDA), total thiol, nitric oxide (NO), and the activity of superoxide dismutase (SOD), along with the concentration of brain-derived neurotrophic factor (BDNF).
Result
Our findings revealed that global I/R injury impaired rats’ performance in the passive avoidance test and increased levels of MDA and NO. Moreover, it decreased the total thiol level, SOD activity, and BDNF level in the hippocampus. Administration of cedrol significantly improved memory function, reduced oxidative stress, NO level and increased BDNF level in the hippocampus.
Conclusion
The results indicate that cedrol has neuroprotective properties in global I/R by reducing oxidative stress and enhancing the levels of BDNF.
Keywords: Cedrol, Global ischemia reperfusion, Memory impairment, Oxidative stress, Herbal remedies
Introduction
Global cerebral ischemia may arise as a consequence of insufficient blood supply for the entire or a significant portion of the brain. Some clinical situations, such as prolonged cardiac arrest can result in global cerebral ischemia, leading to permanent brain damage [1]. When cardiac arrest occurs, blood flow to the brain stops and consciousness is lost almost instantly. If treatment is not received, irreparable brain injury and death will occur quickly [2]. The pathophysiology of brain injury after cardiac arrest can be divided in 2 steps primary injury (minutes to hours), secondary injury (hours to days); The primary injury of cardiac arrest results from occlusion of cerebral blood flow and following depletion of oxygen, glucose, and ATP, as well as a loss of the inner membrane potential in mitochondria. Secondary brain injury may result from ongoing or repeated insufficient oxygen delivery to the brain after blood flow has been partially or fully restored. Hypoxemia, insufficient cerebral perfusion pressure caused by hypotension or disturbed cerebrovascular autoregulation, as well as elevated intracranial pressure as a result of brain oedema are all contributing causes [2].
Despite extensive research, reliable and effective therapeutic approaches to mitigate global ischemia/reperfusion (I/R)-related neuronal damage and promote post- global I/R recovery are still lacking due to the complex nature of the biochemical cascades and neurologic dysfunction involved [3]. Herbal remedies offer a valuable resource for the development of novel therapeutics in various human illnesses. The pathophysiological processes underlying global I/R include oxidative stress and neuroinflammation [4]. The intricate relationship between global I/R pathophysiology and the multifaceted effects of herbal remedies active ingredients suggests the potential of natural medicine in global I/R treatment [5]. Various bioactive components of medicinal plants have been previously reported to mitigate neuronal damages in global I/R. This beneficial impact is widely known to be exerted through suppressing ischemia-induced reactive oxygen species (ROS) generation and neuroinflammation [5]. Terpenes represent the largest and most diverse class of natural products [6]. Sesquiterpenes, are a subclass of terpenes that are comprised from 3 units of isoprenoid [7, 8]. Various sesquiterpenes such as β-Caryophyllene [9], isofuranodiene [10], showed protective effect in animal cerebral ischemia models. Cedrol is a natural sesquiterpene possesses a wide range of biological activities, including analgesic, anti-inflammatory, antioxidant, anti-cancer, and anti-anxiety properties [7, 11–14].
In our previous studies cedrol possess analgesic activity through reduction of inflammation and oxidative stress in the rat models of neuropathic pain and arthritis [7, 11]; Herein, we sought to elucidate the neuroprotective impact of cedrol on memory and biochemical factors in rats after transient global I/R injury.
Materials and methods
Chemicals
Ketamine and xylazine were obtained from Alfasan Pharmaceutical Co. (Woerden, Netherland). Cedrol, Dimethyl sulfoxide (DMSO), ethylenediaminetetraacetic acid (EDTA), 2-thiobarbituric acid (TBA), hydrochloric acid (HCl), trichloroacetic acid (TCA), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and potassium chloride were bought from Sigma-Aldrich (Sigma Aldrich St Louis MO). Nitric oxide (NO) Assay Kit was purchased from Novin Navand Salamat Company, Iran. Brain-derived neurotrophic factor (BDNF) was measured using the rat BDNF Elisa Kit from My BioSource Company, USA.
Animals
Healthy adult male Wistar rats weighing 220 ± 20 g were obtained from the experimental animal center of the Mashhad University of Medical Sciences (MUMS), Mashhad, Iran. The rats were housed in a controlled environment at a temperature of 22–25 °C and a 12 h/12 hr natural light-dark cycle. The rats had free access to food and water. All experimental procedures were conducted in accordance with the Animal Ethics Guidelines of MUMS. All animal experiments were in agreement with the Ethics Committee Acts, Mashhad University of Medical Sciences (MUMS), (ethical code: IR.MUMS.MEDICAL.REC.1399.490).
Induction of transient global ischemia
Rats were anesthetized using a combination of xylazine (10 mg/kg, i.p) and ketamine (100 mg/kg, intraperitoneally (i.p.)). The neck skin was shaved, and cleaned. Afterward, through a midline neck incision, bilateral carotid arteries were exposed. The arteries were then temporarily occluded by small vascular clips. After 20 min, the clamps were released and reperfusion was initiated. The wound was then sutured [15].
Experimental design
In this experimental study, 40 rats were randomly assigned into five groups of eight each. Treatment groups received 7.5 mg/kg, 15 mg/kg, or 30 mg/kg of cedrol i.p., at 0 min following the induction of cerebral I/R injury once daily for one week. The control and global I/R injury groups received the vehicle (saline/ DMSO1%) with the same protocol as the treatment groups.
Passive avoidance test
For assessing learning and memory functions in rats, we performed passive avoidance test. This test conducted in a quiet, dim room. The test apparatus consisted of a dark space and a light space which are connected by a small entry. Before the test, each rat was allowed to explore the rooms and acclimate to the apparatus for two days, with five minutes per day. The training trial began with placing a rat in the light room with the entry closed. After 15 s, the entry was opened, allowing the rat to enter the dark room. Upon entry, the door was shut and the rat received a small electric shock (2 mA, 2 s). After 30 s, the rat was gently removed from the apparatus. The testing trial was conducted 24 h later. Normally, rats that have learned will remember and refrain the dark space where they received the shock. In the testing trial, the rat was put in the light chamber and the entry was opened after 15 s, giving the rat five minutes to explore the entire apparatus. The duration in the dark room and the delay prior to the initial entry were noted [16].
Biochemical tests
After conducting final behavioral tests, rats were deeply scarified with by CO2 inhalation, rats were decapitated, the brains were removed, and the hippocampal tissue was dissected and kept at − 80 °C until use. The animals were euthanized by a competent person with minimum pain, suffering and distress. The tissue samples were homogenized in 0.1 M ice-cold phosphate-buffered saline (PBS, pH 7.4) to obtain 10% homogenate (w/v) [17].
Assessment of hippocampal total thiol levels
The total thiol content in the hippocampus tissue was measured using the Ellman method, with DTNB serving as the reagent. The interaction of thiol groups with DTNB produces a yellow complex that represents a peak absorbance at 412 nm. For this purpose, a mixture of 50 µl of the homogenate and 1 ml of Tris-EDTA buffer (pH 8.6) was made and its absorbance at 412 nm was measured against Tris-EDTA buffer alone labeled R1. Then, 20 µl of a 10 mM DTNB solution was added to R1 and was read for the second time after 15 min labeled R2. The absorbance of the DTNB solution alone was read as a blank (B). The total thiol concentration (µmole/gr tissue) was calculated using the following equation: (R2 - R1 - B) × 1.07/0.05 × 13.6 [18].
Assessment of hippocampal malondialdehyde levels
To measure malondialdehyde (MDA), we provided a mixture of 1 ml of the homogenate sample and 2 ml of the TCA-TBA-HCl reagent, and the solution was heated in a water bath for 45 min. After cooling, the samples were centrifuged for 10 min at 3000 rpm, and the absorbance of the supernatant at 535 nm was measured. The amount of MDA was represented as nmol/g tissue [18].
Assessment of hippocampal NO levels
A rat NO Kit was purchased from Novin Navand Salamat Company, Iran. The hippocampal tissue was used to determine the NO metabolites NO2 and NO3 (Griess reagent method). The Griess reaction was adapted to assay nitrates as previously described [19]. Briefly, The Griess reagent was mixed with the samples. After centrifuging and vortex-mixing the contents, the supernatants were moved to a 96-well microplate. A microplate reader was used to measure absorbance at 520 nm, and the final results were expressed as pg/g tissue [19].
Assessment of hippocampal SOD levels
SOD activity was estimated using the method that Madesh and Balasubramanian outlined [20]. The pyrogallol autoxidation process produces superoxide, and SOD inhibits the superoxide-dependent reduction of the tetrazolium dye to its formazan, resulting in a colorimetric experiment. At 570 nm, the colorimetric changes were measured. The amount of enzyme that causes 50% inhibition in the reduction rate of the tetrazolium dye (MTT) was defined as one unit of SOD activity [21].
Assessment of hippocampal BDNF levels
Anti-BDNF polyclonal antibody was pre-coated on to 96-well plates. The biotin-conjugated anti-BDNF polyclonal antibody was employed as a detection antibody. After adding the standards, test samples, and biotin-conjugated detection antibody to the wells, a wash buffer was used to wash the wells. Avidin-biotin-peroxidase complex was added to the wells, the wells were again washed with buffer to remove the unbound conjugates. Also, 3,3′,5,5′-tetramethylbenzidine (TMB) substrates were used as a color reagent in HRP enzymatic reaction. After adding the acidic stop solution, the TMB substrate catalyzed by HRP generates a blue color product that changes to yellow. The intensity of the yellow color is related to the amount of BDNF present in the sample collected on the plate. Using a microplate reader, the samples’ absorbance at 450 nm was determined, and the BDNF concentration was expressed as pg/g tissue [22].
Statistical analysis
GraphPad Prism software version 8.0 was carried out to perform statistical analysis and graphical presentations. Kolmogorov–Smirnov and Levene’s tests were used for normality or homogeneity, respectively. The data of biochemical data were analyzed using one-way ANOVA and Tukey’s post hoc tests. The data of the passive avoidance test were analyzed using Kruskal–Wallis followed by Dunn’s pairwise comparison test. All data were expressed as mean ± SEM except the data of the passive avoidance test, which were shown as median and interquartile range. P < 0.05 was considered statistically significant.
Results
Passive avoidance test
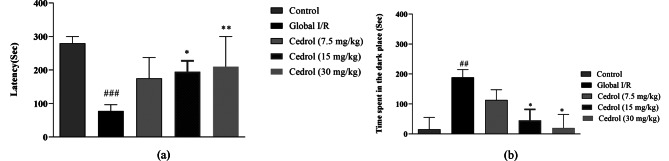
The rats in the global I/R group had less delay time for entering the dark space versus the control group (P < 0.001). Conversely, rats treated with cedrol at doses of 15 and 30 mg/kg demonstrated a longer delay time for going into the dark space compared to the global I/R group (P < 0.05, and P < 0.01, respectively) (Fig. 1A). Furthermore, the rats in the global I/R group spent more time in the dark chamber, versus the control group (P < 0.01). Administration of 15 or 30 mg/kg of cedrol significantly diminished the time spent in the dark chamber, versus the global I/R group 24 hours’ post-shock (P < 0.05, Fig. 1B).
Fig. 1.
Results of the passive avoidance test: (A) the delay before entrance to the dark chamber and (B) the total time duration spent in the dark chamber. The results are expressed as median and interquartile range (n = 8 per group). * P < 0.05 and ** P < 0.01 present the difference between other groups and ischemic group; ## P < 0.01 and ### P < 0.001 versus the control group
Hippocampal oxidative stress level
In the global I/R rats, the hippocampal level of MDA was significantly higher versus the control group (P < 0.001). Cedrol at doses of 7.5, 15, and 30 mg/kg significantly diminished the elevated hippocampal MDA levels when versus the global I/R group (P < 0.05, P < 0.001, and P < 0.001, respectively) (Fig. 2A). Transient global cerebral I/R significantly diminished the total thiol level in the hippocampus, in comparison with the control rats (P < 0.001). In comparison with the global I/R group, injection of 15 mg/kg and 30 mg/kg doses of cedrol significantly enhanced the total thiol levels (P < 0.001) (Fig. 2B). Moreover, SOD activity was significantly diminished in the hippocampus of the global I/R group (P < 0.001) versus the control group. However, treatment with cedrol at doses of 15 mg/kg and 30 mg/kg effectively enhanced SOD activity in the hippocampus (P < 0.01 and P < 0.001, respectively) (Fig. 2C).
Fig. 2.
Effects of cedrol on (A) MDA, (B) thiol, and (C) SOD activity in the hippocampal tissues of the experimental groups. The results are expressed as mean ± SEM. (n = 8 per group). * P < 0.05, ** P < 0.01, and *** P < 0.001 present the difference between other groups and ischemic group; ### P < 0.001 versus the control group. MDA: malondialdehyde, SOD: superoxide dismutase
Hippocampal BDNF level
The concentration of BDNF in the hippocampus showed a significant diminution in the global I/R group (P < 0.001). The results showed that cedrol at a dose of 15 mg/kg and 30 mg/kg was able to increase the hippocampal BDNF level (P < 0.05 and P < 0.001 respectively) (Fig. 3).
Fig. 3.
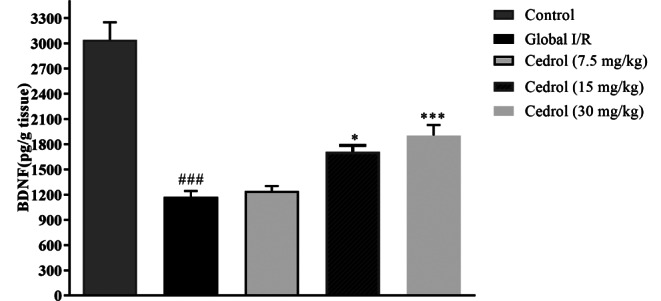
Effect of cedrol on BDNF level in the hippocampal tissues of the experimental groups. The results are expressed as mean ± SEM. (n = 6 per group). * P < 0.05, *** P < 0.001 present the difference between other groups and ischemic group; ### P < 0.01 versus the control group. BDNF: brain-derived neurotrophic factor
Hippocampal NO level
Transient global I/R significantly increased the levels of NO in the hippocampus versus the control group (P < 0.001). The results also showed that cedrol at doses of 15 mg/kg and 30 mg/kg reversed this effect and significantly reduced the hippocampal NO levels compared to the global I/R group (P < 0.01 and P < 0.001, respectively) (Fig. 4).
Fig. 4.

Effect of cedrol on NO level in the hippocampal tissues of the experimental groups. The results are expressed as mean ± SEM. (n = 6 per group). ** P < 0.01 and *** P < 0.001 present the difference between other groups and ischemic group; ### P < 0.001 versus the control group. NO: nitric oxide
Discussion
In this study, we investigated the therapeutic potential of cedrol on memory impairment caused by global I/R injury in rats and the potential mechanisms underlying this impact. Global ischemia can be induced by occluding both common carotid arteries, along with the induction of hypotension for a limited duration. This model, result in damage to the hippocampus, the caudate putamen and neocortex [23]. The reperfusion leads to a severe damage to the previously injured neurons upon the return of blood flow [24]. The hippocampus, crucial for learning and memory, is particularly vulnerable to ischemic damage during global ischemia. Various factors like excitotoxicity, inflammation, and oxidative stress contribute to hippocampal neuronal damage and demise. Some previous studies have indicated that the hippocampus is a central site of pathological changes after brain ischemia, leading to a decline in memory function [25, 26].
To evaluate memory function, we employed the passive avoidance test. This test is commonly used in the field of neuroscience as a primary method for scrutinizing the impact of interventions on memory and learning functions. It is a simple-set up test that can assess various domains of cognitive function. Furthermore, being a single-trial task, this test is advantageous for accurate timing of interventions and distinctive assessment of the effects on different stages of memory formation [27]. Animals with competent memory function typically avoid the dark space due to the shock they took at the training trial [28]. In our study, the rats in the global I/R group spent more time in the dark chamber and entered to this segment more frequently than the control group. Cedrol increased the time it took for the rats to enter the dark chamber. Additionally, cedrol reduced the time spent in the dark. So, it shows that cedrol revealed protective function on memory impairments that caused by global I/R.
BDNF is a neurotrophin that is essential for memory and learning. Learning and memory impairment following cerebral ischemic insults could be partly attributed to reduced hippocampal BDNF expression. BDNF is a downstream target of cAMP-response element binding protein and contributes to neurogenesis, synaptic plasticity, and cell survival [29, 30]. It plays a crucial role in the growth and maturation of the nervous system, aiding in the development of axons, dendrites, and synaptic specializations. BDNF also regulates neurotransmitter systems, influencing neuronal communication and brain function [31]. BDNF plays numerous important roles in the regulation of excitatory and inhibitory neurotransmitters, and neuronal growth. Low levels of BDNF have been found in patients with metabolic syndrome and cerebrovascular disease, and there is a negative relationship between circulating BDNF and blood pressure, body mass index, and lipid profile. Patients with acute stroke have lower BDNF contents than the healthy controls [32].
Herein, we measured BDNF levels in the hippocampal tissue, which were found to be lower in the global I/R rats. Treatment with cedrol could reverse the decline in the BDNF levels.
Oxidative stress also contributes to the damage caused by global I/R. It is characterized by enhanced levels of ROS and reactive nitrogen species that damages vital cellular components. Ischemia-induced increment of oxidative stress damages DNA, triggers pro-death signaling pathways and jeopardizes functional recovery after global I/R [33]. MDA serves as a commonly used oxidative stress biomarker in various illnesses, including global I/R. It results from lipid peroxidation, arising from the breakdown of phospholipids in cellular membranes. MDA is a reliable biomarker of lipid oxidation because it enters the bloodstream after being released into the extracellular space. It can react with proteins and nucleic acids, further damaging cellular components [34, 35]. SOD is the primary enzyme that guards against cellular damage from oxygen radicals. It catalyzes the conversion of superoxide anion free radicals into O2 and H2O2. Diminished SOD activity can lead to oxidative damage [36]. The thiol or sulphydryl (-SH) antioxidant defense mechanisms protect body from ROS damages. Thiols exhibit antioxidant properties through processes such as thiol-disulfide redox buffering, radical quenching, and metal ion chelation. Thiol-disulfide exchange reactions readily occur in the presence of ROS, converting thiol groups into reversible disulfide bonds. This double-sided cycle is crucial for several biological processes, including signal transmission, cell growth, and apoptosis, as well as antioxidant systems [37]. In our study, thiol level, and SOD activity were lower, and MDA level was upper in the global I/R rats. Cedrol reduced oxidative stress, as evidenced by a diminution in the MDA level in the hippocampal tissue and an increase in the hippocampal SOD activity and thiol level. Similar findings have been reported in the neuropathic pain and arthritis models treated with cedrol [7, 11].
NO and global I/R are closely interconnected. Following a stroke, three types of nitric oxide synthases (NOSs) are produced. Endothelial NOS (eNOS)-derived NO exhibits a neuroprotective effect in cerebral ischemia, in contrast to inducible NOS (iNOS)- and neuronal NOS (nNOS)-derived NO, which contribute to neuronal damage. The harmful consequences of NO produced by iNOS and nNOS primarily result from the generation of nitrates and free radicals, directly damaging mitochondrial enzymes and genetic material. In contrast, the neuroprotective properties of NO generated by eNOS primarily involve the regulation of vascular beds [38]. High amounts of NO, produced due to abnormal NOS stimulation during brain I/R, affects several cellular pathways, leading to cell apoptosis, blood-brain barrier functional impairment, and ultimately brain damage [39]. In our study, global I/R injury led to an increase in NO levels, which cedrol was able to decrease.
Previous studies have highlighted the neuroprotective impact of sesquiterpenes in stroke. Acute pre-treatment with 10 mg/kg Isofuranodiene, a natural sesquiterpene, significantly decreased inflammatory markers and MDA. Additionally, Isofuranodiene promoted a faster recovery and improved neurological performance in an animal model of ischemic stroke [10].
Terpenoids fraction from Hygrophila auriculata reduced oxidative stress markers as demonstrated by increment of brain SOD and GSH with a decrement in MDA level against transient global cerebral ischemia [40]. Administration of cynaropicrin, a sesquiterpene lactone, increased antioxidant capacity in a dose-dependent manner. This effect was exerted through upregulation of nuclear factor-E2-related factor 2 (Nrf2), which is main regulator of body’s antioxidant capacity. Cynaropicrin also mitigated nitrate and MDA levels, as well as proinflammatory cytokines in a rat model of focal cerebral I/R injury [41].
Cedrol has also been reported to suppress interleukin-1β-induced apoptosis in chondrocytes [42]. Dehydrocostuslactone, another sesquiterpene lactone, ameliorated oxygen‑glucose deprivation/reoxygenation-induced injury in the rats hippocampal slices by suppressing apoptosis and autophagy [43]. Similarly, in rats with cerebral ischemia, parthenolide, another sesquiterpene lactone, significantly decreased cerebral infarction, neuronal apoptosis, and inflammatory factor levels, and ameliorated neurological impairments [44].
Conclusion
Our findings demonstrate the protective impact of cedrol in global cerebral I/R injury. We concluded that administration of cedrol improved memory in rats with I/R brain damage. These effects could be exerted by reversing the increased level of global I/R -induced oxidative stress and the diminished level of BDNF in the hippocampus. Hence, this herbal treatment could have the potential to be considered as a beneficial therapy for ameliorating global I/R-derived brain damage. Future research may focus on other potential mechanisms of action and the exact cellular and molecular pathways that contribute to the beneficial impact of cedrol in global I/R neuronal damage.
Acknowledgements
This work was supported by a grant (990529) from the Vice-Chancellor for Research and Technology, MUMS, Mashhad, Iran.
Author contributions
F.F. and S.A. designed and supervised the study. A.M. P-SH., F.F., and A.M. A. contributed to conducting the experiments, data collection, and data analysis. F. F, S.A., and A.M. A., contributed to writing. All authors read and approved the final manuscript.
Funding
This research was supported by grants from the Vice Chancellery for Research and Technology, MUMS, Mashhad, Iran. (990529).
Data availability
The datasets of the current study are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by Mashhad University of Medical Sciences (MUMS) Ethics Committee Acts (ethical code: IR.MUMS.MEDICAL.REC.1399.490). This study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke research and treatment. 2013;2013. [DOI] [PMC free article] [PubMed]
- 2.Perkins GD, Callaway CW, Haywood K, Neumar RW, Lilja G, Rowland MJ, et al. Brain injury after cardiac arrest. Lancet. 2021;398(10307):1269–78. [DOI] [PubMed] [Google Scholar]
- 3.Ahmari M, Sharafi A, Mahmoudi J, Jafari-Anarkoli I, Gharbavi M, Hosseini M-J. Selegiline (L-Deprenyl) mitigated oxidative stress, cognitive abnormalities, and histopathological change in rats: alternative therapy in transient global ischemia. J Mol Neurosci. 2020;70:1639–48. [DOI] [PubMed] [Google Scholar]
- 4.Jivad N, Rabiei Z. Review on herbal medicine on brain ischemia and reperfusion. Asian Pac J Trop Biomed. 2015;5(10):789–95. [Google Scholar]
- 5.Behl T, Makkar R, Sehgal A, Sharma N, Singh S, Albratty M, et al. Insights into the explicit protective activity of herbals in management of neurodegenerative and cerebrovascular disorders. Molecules. 2022;27(15):4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox-Georgian D, Ramadoss N, Dona C, Basu C. Therapeutic and medicinal uses of terpenes. Medicinal Plants: Springer; 2019. pp. 333–59. [Google Scholar]
- 7.Forouzanfar F, Pourbagher-Shahri AM, Ghazavi H. Evaluation of Antiarthritic and Antinociceptive Effects of Cedrol in a Rat Model of Arthritis. Oxidative Medicine and Cellular Longevity. 2022;2022. [DOI] [PMC free article] [PubMed]
- 8.Bártíková H, Hanusova V, Skalova L, Ambroz M, Bousova I. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr Top Med Chem. 2014;14(22):2478–94. [DOI] [PubMed] [Google Scholar]
- 9.Chang H-J, Kim J-M, Lee J-C, Kim W-K, Chun HS. Protective effect of β-caryophyllene, a natural bicyclic sesquiterpene, against cerebral ischemic injury. J Med Food. 2013;16(6):471–80. [DOI] [PubMed] [Google Scholar]
- 10.Yousefi-Manesh H, Dehpour AR, Shirooie S, Bagheri F, Farrokhi V, Mousavi SE, et al. Isofuranodiene, a natural sesquiterpene isolated from wild celery (Smyrnium olusatrum L.), protects rats against acute ischemic stroke. Pharmaceuticals. 2021;14(4):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakhaee MH, Sayyadi SAH, Sakhaee N, Sadeghnia HR, Hosseinzadeh H, Nourbakhsh F, et al. Cedrol protects against chronic constriction injury-induced neuropathic pain through inhibiting oxidative stress and inflammation. Metab Brain Dis. 2020;35:1119–26. [DOI] [PubMed] [Google Scholar]
- 12.Wang J-w, Chen S-s, Zhang Y-m, Guan J, Su G-Y, Ding M, et al. Anti-inflammatory and analgesic activity based on polymorphism of cedrol in mice. Environ Toxicol Pharmacol. 2019;68:13–8. [DOI] [PubMed] [Google Scholar]
- 13.Xu C, Jin S-Q, Jin C, Dai Z-H, Wu Y-H, He G-L, et al. Cedrol, a ginger-derived sesquiterpineol, suppresses estrogen-deficient osteoporosis by intervening NFATc1 and reactive oxygen species. Int Immunopharmacol. 2023;117:109893. [DOI] [PubMed] [Google Scholar]
- 14.Chien J-H, Chang K-F, Lee S-C, Lee C-J, Chen Y-T, Lai H-C, et al. Cedrol restricts the growth of colorectal cancer in vitro and in vivo by inducing cell cycle arrest and caspase-dependent apoptotic cell death. Int J Med Sci. 2022;19(13):1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikholeslami MA, Ghafghazi S, Pouriran R, Mortazavi SE, Parvardeh S. Attenuating effect of paroxetine on memory impairment following cerebral ischemia-reperfusion injury in rat: the involvement of BDNF and antioxidant capacity. Eur J Pharmacol. 2021;893:173821. [DOI] [PubMed] [Google Scholar]
- 16.Forqani MA, Akbarian M, Amirahmadi S, Soukhtanloo M, Hosseini M, Forouzanfar F. Carvacrol improved learning and memory and attenuated the brain tissue oxidative damage in aged male rats. Int J Neurosci. 2023; 134(11):1–8. [DOI] [PubMed]
- 17.Rakhshandeh H, Ghorbanzadeh A, Negah SS, Akaberi M, Rashidi R, Forouzanfar F. Pain-relieving effects of Lawsonia inermis on neuropathic pain induced by chronic constriction injury. Metab Brain Dis. 2021;36:1709–16. [DOI] [PubMed] [Google Scholar]
- 18.Rakhshandeh H, Pourbagher-Shahri AM, Hasanpour M, Iranshahi M, Forouzanfar F. Effects of Capparis Spinosa extract on the neuropathic pain induced by chronic constriction injury in rats. Metab Brain Dis. 2022;37(8):2839–52. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini M, Dastghaib SS, Rafatpanah H, Hadjzadeh MA-R, Nahrevanian H, Farrokhi I. Nitric oxide contributes to learning and memory deficits observed in hypothyroid rats during neonatal and juvenile growth. Clinics. 2010;65(11):1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madesh M, Balasubramanian K. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35(3):184–8. [PubMed] [Google Scholar]
- 21.Mokhtari-Zaer A, Hosseini M, Salmani H, Arab Z, Zareian P. Vitamin D(3) attenuates lipopolysaccharide-induced cognitive impairment in rats by inhibiting inflammation and oxidative stress. Life Sci. 2020;253:117703. [DOI] [PubMed] [Google Scholar]
- 22.Mansouri F, Ghanbari H, Marefati N, Arab Z, Salmani H, Beheshti F, et al. Protective effects of vitamin D on learning and memory deficit induced by scopolamine in male rats: the roles of brain-derived neurotrophic factor and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:1451–66. [DOI] [PubMed] [Google Scholar]
- 23.Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part two: modeling cerebral ischemia. open Neurol J. 2010;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatemi I, Askari PS, Hakimizadeh E, Kaeidi A, Moghaddam SE, Pak-Hashemi M, et al. Chronic treatment with coenzyme Q10 mitigates the behavioral dysfunction of global cerebral ischemia/reperfusion injury in rats. Iran J Basic Med Sci. 2022;25(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. The anatomical record: advances in integrative anatomy and Evolutionary Biology: advances in integrative anatomy and Evolutionary Biology. 2009;292(12):1914–21. [DOI] [PubMed]
- 26.El Khashab IH, Abdelsalam RM, Elbrairy AI, Attia AS. Chrysin attenuates global cerebral ischemic reperfusion injury via suppression of oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2019;112:108619. [DOI] [PubMed] [Google Scholar]
- 27.Ögren S, Stiedl O. Passive avoidance. Encyclopedia Psychopharmacol. 2010;2:960–7. [Google Scholar]
- 28.Luvisetto S, Basso E, Petronilli V, Bernardi P, Forte M. Enhancement of anxiety, facilitation of avoidance behavior, and occurrence of adult-onset obesity in mice lacking mitochondrial cyclophilin D. Neuroscience. 2008;155(3):585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng C-Y, Kao S-T, Lee Y-C. Angelica Sinensis extract protects against ischemia-reperfusion injury in the hippocampus by activating p38 MAPK-mediated p90RSK/p-Bad and p90RSK/CREB/BDNF signaling after transient global cerebral ischemia in rats. J Ethnopharmacol. 2020;252:112612. [DOI] [PubMed] [Google Scholar]
- 30.Lee S-H, Ko I-G, Kim S-E, Hwang L, Jin J-J, Choi H-H, et al. Aqueous extract of Cordyceps alleviates cerebral ischemia-induced short-term memory impairment in gerbils. J Exerc Rehabilitation. 2016;12(2):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correia AS, Cardoso A, Vale N. BDNF unveiled: exploring its role in Major Depression Disorder Serotonergic Imbalance and Associated stress conditions. Pharmaceutics. 2023;15(8):2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karantali E, Kazis D, Papavasileiou V, Prevezianou A, Chatzikonstantinou S, Petridis F, et al. Serum BDNF levels in acute stroke: a systematic review and meta-analysis. Medicina. 2021;57(3):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu W, et al. Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menon B, Ramalingam K, Kumar R. Evaluating the role of oxidative stress in acute ischemic stroke. J Neurosciences Rural Pract. 2020;11(01):156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abd-Elsameea A, Moustaf A, Mohamed A. Modulation of the oxidative stress by metformin in the cerebrum of rats exposed to global cerebral ischemia and ischemia/reperfusion. Eur Rev Med Pharmacol Sci. 2014;18(16). [PubMed]
- 36.Feng S, Yang M, Liu S, He Y, Deng S, Gong Y. Oxidative stress as a bridge between age and stroke: a narrative review. J Intensive Med. 2023; 3(4): 313-319. [DOI] [PMC free article] [PubMed]
- 37.Çakırca G, Damar Çakırca T, Üstünel M, Torun A, Koyuncu I. Thiol level and total oxidant/antioxidant status in patients with COVID-19 infection. Irish Journal of Medical Science (1971-). 2021:1–6. [DOI] [PMC free article] [PubMed]
- 38.Chen Z-q, Mou R-t, Feng D-x, Wang Z, Chen G. The role of nitric oxide in stroke. Med Gas Res. 2017;7(3):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Hong F, Yang S. Roles of nitric oxide in brain ischemia and reperfusion. Int J Mol Sci. 2022;23(8):4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanhere R, Anjana A, Anbu J, Sumithra M. Neuroprotective and antioxidant potential of terpenoid fraction from Hygrophila auriculata against transient global cerebral ischemia in rats. Pharm Biol. 2013;51(2):181–9. [DOI] [PubMed] [Google Scholar]
- 41.Jin T, Leng B. Cynaropicrin averts the oxidative stress and neuroinflammation in ischemic/reperfusion injury through the modulation of NF-kB. Appl Biochem Biotechnol. 2023; 195(9):5424-5428. [DOI] [PMC free article] [PubMed]
- 42.Dong W, Wang S, Qian W, Li S, Wang P. Cedrol alleviates the apoptosis and inflammatory response of IL-1β-treated chondrocytes by promoting mir-542-5p expression. Vitro Cell Dev Biology-Animal. 2021;57:962–72. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Q, Chen A, Wang X, Zhang Z, Zhao Y, Huang Y, et al. Protective effects of dehydrocostuslactone on rat hippocampal slice injury induced by oxygen–glucose deprivation/reoxygenation. Int J Mol Med. 2018;42(2):1190–8. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Miao L, Peng Q, Fan X, Song W, Yang B, et al. Parthenolide modulates cerebral ischemia-induced microglial polarization and alleviates neuroinflammatory injury via the RhoA/ROCK pathway. Phytomedicine. 2022;105:154373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author on request.