Abstract
Objective
This study investigates the association between the Weekend Warrior (WW) pattern and the risk of anxiety among American adults, offering insights into a more flexible exercise strategy for individuals with limited time for regular exercise during weekdays.
Methods
We analyzed data from the 2007–2012 National Health and Nutrition Examination Survey (NHANES) to examine the relationship between different physical activity (PA) patterns and the risk of anxiety. Multivariate logistic regression, subgroup interaction, restricted cubic spline analysis (RCS), and sensitivity analyses were conducted to assess this association.
Results
Compared to inactive individuals, those engaging in WW pattern (OR = 0.65, 95% CI: 0.48–0.90, p = 0.010), insufficiently active (OR = 0.71, 95% CI: 0.62–0.82, p < 0.001), or regularly active pattern (OR = 0.75, 95% CI: 0.65–0.87, p < 0.001) showed significantly lower risk of anxiety. Subgroup interaction analyses revealed significant effect modification in the poverty income ratio (PIR) and diabetes subgroups (P for interaction < 0.05), while no significant interactions were observed for other variables. RCS analysis showed a significant nonlinear relationship between recreational moderate to vigorous intensity physical activity and risk of anxiety (P for nonlinear < 0.001). Sensitivity analyses further confirmed the stability of the findings.
Conclusion
The WW pattern was associated with a lower risk of anxiety. For individuals unable to exercise consistently throughout the week, the WW pattern offers a practical alternative for reducing the risk of anxiety, particularly among those with lower income levels or diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-025-06612-x.
Keywords: Anxiety, Weekend Warrior, Physical activity patterns, NHANES
Introduction
According to the Eleventh Revision of the International Classification of Diseases and Related Health Problems (ICD-11), the general symptoms of anxiety disorders are characterized by persistent worry, tension, and fear, accompanied by autonomic nervous system responses (such as palpitations, sweating, and trembling), avoidant behaviors, and emotional irritability and restlessness, which cause significant impairment in the individual’s daily life, work, or social functioning [1]. A comprehensive systematic review revealed significant global variation in the prevalence of anxiety disorders among adults, ranging from 3.8 to 25% across different regions and populations [2]. Women are about 1.7 times more likely than men to experience anxiety disorders, as evidenced by a study of 20,013 Americans showing significantly higher lifetime prevalence in women [3]. Anxiety disorders markedly diminish patients’ quality of life, significantly impairing social, occupational, and physical functioning [4]. Currently, pharmacological treatments, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and benzodiazepines, alongside cognitive behavioral therapy, are considered primary options for the management of anxiety disorders [5–8]. However, the practical issue of these treatments and the economic burden they impose hinder their widespread implementation in low and middle-income regions [9]. Identifying more feasible and beneficial interventions can enhance the management of anxiety disorders.
Physical activity (PA) interventions have emerged as a viable and evidence-based therapeutic strategy for mitigating the symptoms of anxiety disorders [10, 11]. Specifically, most research on PA interventions for anxiety disorders has predominantly centered on the benefits of regular PA, typically defined as engaging in exercise three or more sessions per week [12–14]. Given the fast-paced nature of modern work and life, maintaining a routine of three or more exercise sessions per week may present practical challenges in real-world settings. The Weekend Warrior (WW) pattern involves 1 to 2 weekly sessions of PA, with the total weekly volume meeting current PA guidelines, such as 150 min of moderate-intensity exercise or 75 min of vigorous-intensity exercise per week [15]. The WW pattern may represent a time-efficient and health-promoting alternative for individuals seeking the benefits of PA within the constraints of a busy lifestyle. The WW pattern has been linked to reduced cardiovascular disease [16], lower all-cause and cancer mortality risks [17, 18], improved obesity-related outcomes [19], and decreased depression incidence [20]. Nevertheless, existing literature on the association between the WW pattern and anxiety remains limited.
This study aims to investigate the association between the WW pattern and risk of anxiety using the National Health and Nutrition Examination Survey (NHANES) database. Through a comprehensive analysis of relevant data from this dataset, we will assess the relationship between the WW pattern and the risk of anxiety, thereby offering essential insights into the impact of PA on anxiety risk.
Methods
Study population
The data, analyzed in our study, were collected from the NHANES cycles 2007–2008, 2009–2010, and 2011–2012. NHANES is a nationwide, representative survey in the U.S. that assesses the health status of individuals across all age groups and ethnicities. This high-quality survey includes data on demographics, dietary habits, health examinations, laboratory results, and questionnaire responses (https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES research procedure received approval from the Institutional Review Board at the National Center for Health Statistics, and written informed consent was availed.
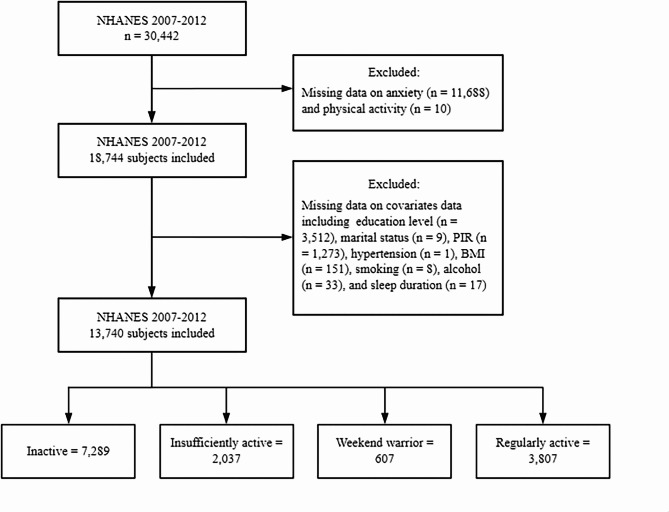
The details of our study’s sampling and exclusion criteria are illustrated in Fig. 1. Initially, a total of 30,442 individuals from the American population were included across the 2007–2012 NHANES cycles. Our analysis first excluded participants who did not respond to the anxiety question (n = 11,688). Additionally, individuals missing recreational PA data were also excluded (n = 10). Subsequently, we adjusted for missing data across all covariates, including education level (n = 3,512), marital status (n = 9), poverty-to-income ratio (PIR) (n = 1,273), hypertension (n = 1), body mass index (BMI) (n = 151), smoking status (n = 8), alcohol status (n = 33), and sleep duration (n = 17). Ultimately, our analysis comprised 13,740 American adults aged over 20. Furthermore, we categorized the eligible participants into four distinct groups: the inactive group (n = 7,289), the insufficiently active group (n = 2,037), the WW group (n = 607), and the regularly active group (n = 3,807).
Fig. 1.
Flow chart of the sample selection process
Assessment of weekend warrior
PA was measured through the Global Physical Activity Questionnaire (GPAQ), in which participants were asked about the frequency and duration of vigorous and moderate-intensity sports, fitness, and recreational activities lasting at least 10 continuous minutes during a typical week [21]. According to the Physical Activity Guidelines for Americans, one minute of vigorous-intensity PA is equivalent to two minutes of moderate-intensity PA [22]. Thus, we calculated total leisure-time moderate to vigorous intensity physical activity (MVPA) by multiplying the minutes of vigorous-intensity PA by two and then adding the minutes of moderate-intensity PA.
Based on The Physical Activity Guidelines for Americans, adults should engage in at least 150 min to 300 min of moderate-intensity aerobic PA per week, or 75 min to 150 min of vigorous-intensity aerobic PA per week, or an equivalent combination of moderate- and vigorous-intensity PA [22]. Four distinct patterns of PA were categorized based on the total duration and frequency of recreational PA in our study [20, 23]. Participants were classified as “inactive” when their MVPA amounted to zero. Individuals were labeled as “insufficiently active” if their MVPA was less than 150 min per week. Those who met or exceeded 150 min of MVPA weekly were categorized as either “weekend warriors” (exercising 1 or 2 times per week) or “regularly active” (exercising at least 3 times per week).
Assessment of anxiety
Anxiety status was assessed through questionnaires based on participants’ responses, as indicated by previous studies [24, 25]. In the NHANES 2007–2012 cycles, anxiety-related information was collected during physical examinations at the Mobile Examination Center using a Computer-Assisted Personal Interviewing system administered by trained interviewers. Participants were posed the following question by a trained physician: “During the past 30 days, for about how many days felt worried, tense, or anxious?” Responses were categorized as follows: those reporting 0 to 6 days were classified as not experiencing anxiety, while those reporting 7 to 30 days were diagnosed with anxiety [26]. This assessment is based on the 14-item Healthy Days measure developed by the United States Centers for Disease Control and Prevention, included in the Health-Related Quality of Life survey, and shows moderate to excellent reliability [27].
Assessment of covariates
The covariates in our study encompassed various demographic factors, including gender (male/female), and age (assessed both as a continuous variable and categorized into three groups: 20–40 years, 40–60 years, and ≥ 60 years). Racial categories included Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races. Education levels were classified as less than 9th grade, 9th-11th grade (including 12th grade without a diploma), high school graduate or GED (General Educational Development certificate), undergraduate, and graduate. Income levels were classified based on the federal poverty income ratio (PIR), which is calculated by dividing family (or individual) income by the poverty guidelines specific to the survey year. Low income was defined as PIR < 1, middle income as PIR 1–3, and high income as PIR ≥ 3. Body mass index (BMI) was categorized into three groups: normal or underweight (< 25 kg/m²), overweight (≥ 25 and < 30 kg/m²), and obese (≥ 30 kg/m²).
Participants were categorized as smokers or non-smokers based on whether they reported having smoked at least 100 cigarettes in their lifetime. Similarly, individuals were classified as drinkers or non-drinkers depending on their answers regarding alcohol consumption, specifically if they had consumed alcoholic beverages at least 12 times within a year. Sleep duration was classified into three categories: short duration (less than 7 h), normal duration (ranging from 7 to 9 h), and long duration (9 h or more). Marital status was classified into two categories: “Married or living with a partner” and “Single,” the latter encompassing individuals who are either widowed, divorced, separated, or have never married. Hypertension was defined based on self-reported diagnoses and/or objective blood pressure measurements, with hypertension being classified if either systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg (average of three readings) was observed. If either of these criteria was met, participants were classified as hypertensive. Individuals were deemed to have diabetes if they met any of the following criteria: (1) fasting plasma glucose (FPG) levels of 126 mg/dL or higher; (2) glycol hemoglobin (HbA1c) of 6.5% or greater; or (3) a self-reported diabetes diagnosis from a healthcare professional.
Statistical analysis
Statistical analyses were conducted using R software, version 4.3.3 (R Project for Statistical Computing), following the guidelines established by the Centers for Disease Control and Prevention and incorporating the appropriate NHANES sampling weights. Continuous variables were summarized as means ± standard deviation (SD), while categorical variables were represented by counts and percentages (%). For continuous variables, a weighted Wilcoxon rank-sum test was applied for complex survey samples, while categorical variables were analyzed using a chi-squared test with Rao & Scott’s second-order correction. Three weighted logistic regression models were developed to evaluate odds ratios (ORs) along with their corresponding 95% confidence intervals (CIs) to explore the relationships between various PA patterns and risk of anxiety. Model 1 was executed without covariate adjustments, while Model 2 adjusted for gender, age, race, education level, marital status, and PIR, and Model 3 further incorporated adjustments for BMI, smoking, alcohol consumption, sleep duration, hypertension, and diabetes. Trend tests were performed across all three models. Subgroup analyses and interaction tests were conducted to examine potential modifications of this association by covariates within the fully adjusted model. Restricted cubic splines (RCS) were employed to investigate the non-linear relationship between total recreational MVPA and risk of anxiety. Sensitivity analyses utilized both unweighted logistic regression and inverse probability treatment weighting (IPTW) logistic regression. Considering the comorbidity of anxiety and depression, we conducted an additional sensitivity analysis by including depression as a covariate in the models. Depression was assessed using the 9-item Patient Health Questionnaire (PHQ-9), with a score of ≥ 10 indicating clinically depression. Specifically, we performed survey-weighted logistic regression controlling for depression and further conducted unweighted and IPTW logistic regression analyses to examine the robustness of the findings. A two-sided p-value of less than 0.05 was considered statistically significant in our analysis.
Results
Baseline characteristics of participants
Among the 13,740 American adults aged 20 to 80 years included from three NHANES cycles, 3,548 individuals (25.82%) were classified as having anxiety based on self-reported questionnaire data. Table 1 presents the baseline characteristics of the study participants, comparing those with anxiety to those without. Individuals with anxiety were more likely to be female, younger, non-Hispanic White, and classified as lower-middle income. They were more likely to be obese and also more likely to be single. Additionally, they were more likely to smoke, consume alcohol, experience shorter sleep durations, and engage in less recreational PA compared to their non-anxious counterparts.
Table 1.
Baseline characteristics of the study population
| Characteristic | Overall (n = 13,740) | Anxiety | P-value | |
|---|---|---|---|---|
| No (n = 10,192) | Yes (n = 3,548) | |||
| Gender, n(%) | < 0.001 | |||
| Male | 6,831 (48.84) | 5,396 (51.53) | 1,435 (41.20) | |
| Female | 6,909 (51.16) | 4,796 (48.47) | 2,113 (58.80) | |
| Age (years, mean ± SD) | 47.04 ± 16.75 |
47.76 ± 17.17 |
44.98 ± 15.32 | < 0.001 |
| Race/ethnicity, n (%) | 0.010 | |||
| Mexican American | 2,011 (7.65) | 1,507 (7.88) | 504 (7.00) | |
| Other Hispanic | 1,361 (5.10) | 952 (4.79) | 409 (5.99) | |
| Non-Hispanic White |
6,406 (70.31) |
4,653 (69.94) | 1,753 (71.36) | |
| Non-Hispanic Black | 2,891 (10.72) | 2,214 (10.83) | 677 (10.41) | |
| Other Races | 1,071 (6.22) | 866 (6.56) | 205 (5.25) | |
| Education level, n (%) | < 0.001 | |||
| Less than 9th grade | 1,434 (5.39) | 1,066 (5.40) | 368 (5.38) | |
| 9−11th grade (Includes 12th grade with no diploma) | 2,150 (12.04) | 1,479 (10.87) | 671 (15.34) | |
| High school graduate or general educational development certificate | 3,168 (22.61) | 2,403 (22.90) | 765 (21.79) | |
| Undergraduate | 3,931 (30.97) | 2,859 (30.20) | 1,072 (33.15) | |
| Graduate | 3,057 (28.98) | 2,385 (30.62) | 672 (24.33) | |
| PIR, n (%) | < 0.001 | |||
| < 1 | 3,004 (14.69) | 2,004 (12.99) | 1,000 (19.53) | |
| [1, 3) | 5,758 (35.64) | 4,256 (34.81) | 1,502 (38.01) | |
| ≥ 3 | 4,978 (49.67) | 3,932 (52.21) | 1,046 (42.46) | |
| BMI, n (%) | 0.008 | |||
| < 25 |
4,006 (30.66) |
2,993 (30.54) |
1,013 (31.01) | |
| [25,30) |
4,589 (33.89) |
3,531 (34.70) |
1,058 (31.58) | |
| ≥ 30 |
5,145 (35.45) |
3,668 (34.76) |
1,477 (37.41) | |
| Marital status, n (%) | < 0.001 | |||
| Married or living with partner | 8,103 (63.12) | 6,161 (64.47) | 1,942 (59.28) | |
| Widowed, divorced, separated or never married | 5,637 (36.88) | 4,031 (35.53) | 1,606 (40.72) | |
| Smoking status, n (%) | < 0.001 | |||
| No | 7,379 (54.42) | 5,672 (56.47) | 1,707 (48.60) | |
| Yes | 6,361 (45.58) | 4,520 (43.53) | 1,841 (51.40) | |
| Alcohol status, n (%) | 0.047 | |||
| No | 3,741 (22.06) | 2,845 (22.64) | 896 (20.41) | |
| Yes | 9,999 (77.94) | 7,347 (77.36) | 2,652 (79.59) | |
| Sleep duration, n (%) | < 0.001 | |||
| < 7 h | 5,463 (36.78) | 3,728 (33.94) | 1,735 (44.85) | |
| [7,9) hours | 7,253 (56.38) | 5,692 (59.37) | 1,561 (47.88) | |
| ≥ 9 h | 1,024 (6.85) | 772 (6.70) | 252 (7.27) | |
| Hypertension, n (%) | 0.479 | |||
| No | 7,875 (63.01) | 5,856 (63.23) | 2,019 (62.37) | |
| Yes | 5,865 (36.99) | 4,336 (36.77) | 1,529 (37.63) | |
| Diabetes, n (%) | 0.234 | |||
| No | 11,457 (88.01) | 8,534 (88.30) | 2,923 (87.20) | |
| Yes | 2,283 (11.99) | 1,658 (11.70) | 625 (12.80) | |
| Pattern of physical activity, n(%) | < 0.001 | |||
| Inactive | 7,289 (46.08) | 5,184 (43.73) | 2,105 (52.75) | |
| Insufficiently active | 2,037 (16.53) | 1,566 (17.24) | 471 (14.51) | |
| Weekend warrior | 607 (5.15) | 483 (5.52) | 124 (4.10) | |
| Regularly active | 3,807 (32.24) | 2,959 (33.51) | 848 (28.64) | |
BMI, body mass index; PIR, Ratio of family income to poverty
Association between physical activity patterns and risk of anxiety
The results from three weighted logistic regression analyses (Table 2) indicate that individuals classified as insufficiently active, WW, and regularly active show a significantly lower risk of anxiety compared to those identified as inactive. Using the inactive group as the reference category, the ORs of anxiety and 95% CIs for the insufficiently active group across the three models were as follows: 0.70 (0.61, 0.80), 0.69 (0.60, 0.79), and 0.71 (0.62, 0.82), all p < 0.001. For the WW group, the ORs of anxiety and 95% CIs were reported as 0.62 (0.46, 0.83), p = 0.002; 0.63 (0.46, 0.86), p = 0.005; and 0.65 (0.48, 0.90), p = 0.010. Meanwhile, regularly active individuals showed ORs and 95% CIs of 0.71 (0.62, 0.81), 0.72 (0.62, 0.83), and 0.75 (0.65, 0.87), all p < 0.001. These findings underscore the association between higher levels of recreational PA and a decreased likelihood of experiencing anxiety.
Table 2.
Association between the weekend warrior and anxiety by survey-weighted logistic regression models, NHANES 2007–2012 (N = 13,740)
| Case/Participants | Model 1 OR (95% CI) |
P-value | Model 2 OR (95% CI) |
P-value | Model 3 OR (95% CI) |
P-value | |
|---|---|---|---|---|---|---|---|
| Compared to inactive | |||||||
| Inactive | 2,105/7,289 | 1 [Reference] | - | 1 [Reference] | - | 1 [Reference] | - |
| Insufficiently active | 471/2,037 | 0.70 (0.61, 0.80) | < 0.001 | 0.69 (0.60, 0.79) | < 0.001 | 0.71 (0.62, 0.82) | < 0.001 |
| Weekend warrior | 124/607 | 0.62 (0.46, 0.83) | 0.002 | 0.63 (0.46, 0.86) | 0.005 | 0.65 (0.48, 0.90) | 0.010 |
| Regularly active | 848/3,807 | 0.71 (0.62, 0.81) | < 0.001 | 0.72 (0.62, 0.83) | < 0.001 | 0.75 (0.65, 0.87) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | 0.002 | ||||
Model 1: no covariates were adjusted
Model 2: gender, age, race/ethnicity, education level, marital status and PIR were adjusted
Model 3: gender, age, race/ethnicity, education level, marital status, PIR, BMI, smoking status, alcohol status, sleep duration, hypertension and diabetes were adjusted
Subgroup analysis
In the subgroup interaction analysis (Table 3), no significant interactions were found for gender, age group, race, education level, marital status, BMI, smoking, alcohol consumption, sleep duration, or hypertension, as all p-values for interaction exceeded 0.05. In contrast, significant interactions were observed for PIR (p for interaction = 0.018) and diabetes status (p for interaction = 0.030). Specifically, the WW exercise pattern was significantly associated with a lower risk of anxiety among individuals with low to middle income and those diagnosed with diabetes. Conversely, this association was non-significant in high-income populations and among individuals without diabetes.
Table 3.
Subgroup analyses assessing the associations between physical activity patterns and anxiety, with “inactive” participants as reference
| Characteristics | Inactive | Insufficiently active | Weekend warrior | Regularly active | P for interaction |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Gender | 0.387 | ||||
| Male | ref | 0.70 (0.55, 0.88) | 0.76 (0.54, 1.07) | 0.86 (0.75, 0.98) | |
| Female | ref | 0.73 (0.61, 0.88) | 0.58 (0.36, 0.93) | 0.72 (0.58, 0.89) | |
| Age group | 0.141 | ||||
| 20–40 years | ref | 0.69 (0.57, 0.83) | 0.60 (0.43, 0.83) | 0.93 (0.76, 1.13) | |
| 40–60 years | ref | 0.79 (0.63, 0.98) | 0.81 (0.47, 1.39) | 0.65 (0.51, 0.82) | |
| ≥ 60 years | ref | 0.64 (0.49, 0.85) | 0.99 (0.39, 2.52) | 0.75 (0.54, 1.03) | |
| Race | 0.453 | ||||
| Mexican American | ref | 0.67 (0.48, 0.93) | 1.00 (0.59, 1.69) | 0.69 (0.51, 0.94) | |
| Other Hispanic | ref | 0.70 (0.48, 1.02) | 0.28 (0.10, 0.75) | 0.77 (0.58, 1.02) | |
| Non-Hispanic White | ref | 0.68 (0.58, 0.80) | 0.67 (0.45, 0.98) | 0.76 (0.64, 0.91) | |
| Non-Hispanic Black | ref | 0.97 (0.74, 1.27) | 0.73 (0.45, 1.21) | 0.91 (0.75, 1.11) | |
| Other Races | ref | 0.96 (0.49, 1.88) | 1.07 (0.47, 2.44) | 0.83 (0.51, 1.34) | |
| Education level | 0.688 | ||||
| Less than 9th grade | ref | 0.75 (0.42, 1.34) | 0.74 (0.25, 2.23) | 0.75 (0.44, 1.29) | |
| 9−11th grade (Includes 12th grade with no diploma) | ref | 0.80 (0.54, 1.20) | 0.61 (0.28, 1.30) | 0.64 (0.44, 0.92) | |
| High school graduate or general educational development certificate | ref | 0.81 (0.61, 1.08) | 0.50 (0.28, 0.89) | 0.72 (0.55, 0.93) | |
| Undergraduate | ref | 0.61 (0.48, 0.78) | 0.78 (0.47, 1.29) | 0.78 (0.60, 1.00) | |
| Graduate | ref | 0.82 (0.60, 1.11) | 0.83 (0.48, 1.42) | 0.90 (0.72, 1.13) | |
| Marital status | 0.275 | ||||
| Married or living with partner | ref | 0.69 (0.57, 0.83) | 0.82 (0.55, 1.24) | 0.80 (0.68, 0.95) | |
| Widowed, divorced, separated or never married | ref | 0.78 (0.64, 0.95) | 0.52 (0.34, 0.78) | 0.74 (0.62, 0.89) | |
| PIR | 0.018 | ||||
| < 1 | ref | 0.83 (0.64, 1.09) | 0.52 (0.33, 0.83) | 0.68 (0.54, 0.85) | |
| [1, 3) | ref | 0.61 (0.49, 0.77) | 0.54 (0.37, 0.79) | 0.59 (0.48, 0.72) | |
| ≥ 3 | ref | 0.82 (0.62, 1.08) | 0.93 (0.60, 1.43) | 1.00 (0.81, 1.23) | |
| BMI | 0.499 | ||||
| < 25 | ref | 0.77 (0.59, 1.00) | 0.76 (0.48, 1.19) | 0.79 (0.65, 0.95) | |
| [25, 30) | ref | 0.57 (0.42, 0.77) | 0.75 (0.48, 1.16) | 0.76 (0.62, 0.92) | |
| ≥ 30 | ref | 0.82 (0.62, 1.08) | 0.56 (0.33, 0.94) | 0.79 (0.62, 1.00) | |
| Smoking status | 0.060 | ||||
| No | ref | 0.75 (0.62, 0.91) | 0.90 (0.62, 1.31) | 0.89 (0.73, 1.07) | |
| Yes | ref | 0.70 (0.58, 0.84) | 0.53 (0.37, 0.77) | 0.68 (0.57, 0.81) | |
| Alcohol status | 0.313 | ||||
| No | ref | 0.75 (0.56, 1.00) | 0.96 (0.48, 1.92) | 0.96 (0.74, 1.24) | |
| Yes | ref | 0.71 (0.61, 0.82) | 0.65 (0.47, 0.89) | 0.74 (0.64, 0.86) | |
| Sleep duration | 0.728 | ||||
| < 7 h | ref | 0.61 (0.46, 0.81) | 0.63 (0.45, 0.89) | 0.74 (0.61, 0.90) | |
| [7,9) hours | ref | 0.79 (0.64, 0.97) | 0.70 (0.44, 1.14) | 0.80 (0.66, 0.97) | |
| ≥ 9 h | ref | 0.93 (0.54, 1.61) | 1.13 (0.36, 3.55) | 0.89 (0.61, 1.30) | |
| Hypertension | 0.526 | ||||
| No | ref | 0.71 (0.60, 0.86) | 0.71 (0.51, 0.98) | 0.83 (0.70, 0.98) | |
| Yes | ref | 0.74 (0.58, 0.93) | 0.66 (0.37, 1.17) | 0.68 (0.53, 0.87) | |
| Diabetes | 0.030 | ||||
| No | ref | 0.75 (0.66, 0.87) | 0.73 (0.53, 1.00) | 0.82 (0.71, 0.94) | |
| Yes | ref | 0.53 (0.38, 0.75) | 0.29 (0.11, 0.74) | 0.50 (0.32, 0.76) |
BMI, body mass index; PIR, Ratio of family income to poverty
All models were adjusted for gender, age group, race, education level, marital status, PIR, BMI, smoking, alcohol, sleep duration, hypertension, and diabetes
Nonlinear association between recreational MVPA and the risk of anxiety
Multivariable-adjusted RCS regression was employed to explore the dose-response relationship between recreational MVPA and the risk of anxiety (Fig. 2). A non-linear association was observed (p for nonlinearity < 0.001), with the RCS curve demonstrating a U-shaped trajectory. Although the OR exceeded 1 at levels of MVPA beyond 2,431 min per week, this increase in risk of anxiety was not statistically significant. These findings indicate that both inactive and excessive recreational MVPA are associated with a higher risk of anxiety.
Fig. 2.
Dose-response relationship between recreational moderate-to-vigorous intensity physical activity and anxiety
Sensitivity analysis
In the sensitivity analyses, both unweighted logistic regression and IPTW logistic regression were conducted. In both models, compared to the inactive group, the insufficiently active, WW, and regularly active PA patterns were associated with a lower risk of anxiety. The results remained consistent with those obtained from our multivariable weighted logistic regression analysis (Table 4). Given the potential comorbidity between anxiety and depression, we conducted an additional sensitivity analysis including depression as a covariate. Using the Inactive group as the reference, the survey-weighted logistic regression model (Table S1) showed that the association between the WW pattern and anxiety was not statistically significant (OR = 0.78, 95% CI: 0.56–1.08, p = 0.125). However, in unweighted (OR = 0.79, 95% CI: 0.63–0.98, p = 0.038) and IPTW (OR = 0.79, 95% CI: 0.63–0.99, p = 0.042) analyses (Table S2), the association remained statistically significant.
Table 4.
Association between physical activity patterns and anxiety: a comparison of unweighted and IPTW logistic regression
| Model 1 OR (95% CI) |
P-value | Model 2 OR (95% CI) |
P-value | Model 3 OR (95% CI) |
P-value | |
|---|---|---|---|---|---|---|
| Compared to inactive, unweighted | ||||||
| Inactive | 1 [Reference] | - | 1 [Reference] | - | 1 [Reference] | - |
| Insufficiently active | 0.74 (0.66, 0.83) | < 0.001 | 0.73 (0.65, 0.82) | < 0.001 | 0.75 (0.66, 0.84) | < 0.001 |
| Weekend warrior | 0.63 (0.51, 0.77) | < 0.001 | 0.64 (0.52, 0.79) | < 0.001 | 0.67 (0.54, 0.83) | < 0.001 |
| Regularly active | 0.71 (0.64, 0.77) | < 0.001 | 0.70 (0.64, 0.78) | < 0.001 | 0.74 (0.67, 0.82) | < 0.001 |
| Compared to inactive, IPTW | ||||||
| Inactive | 1 [Reference] | - | 1 [Reference] | - | 1 [Reference] | - |
| Insufficiently active | 0.74 (0.66, 0.83) | < 0.001 | 0.73 (0.65, 0.82) | < 0.001 | 0.75 (0.66, 0.84) | < 0.001 |
| Weekend warrior | 0.63 (0.52, 0.78) | < 0.001 | 0.64 (0.52, 0.80) | < 0.001 | 0.67 (0.54, 0.84) | < 0.001 |
| Regularly active | 0.71 (0.64, 0.77) | < 0.001 | 0.70 (0.64, 0.78) | < 0.001 | 0.74 (0.67, 0.82) | < 0.001 |
Model 1: no covariates were adjusted
Model 2: gender, age, race/ethnicity, education level, marital status and PIR were adjusted
Model 3: gender, age, race/ethnicity, education level, marital status, PIR, BMI, smoking status, alcohol status, sleep duration, hypertension and diabetes were adjusted
IPTW, inverse probability of treatment weighting
Discussion
Our findings indicated that the WW pattern, along with insufficiently active and regularly active patterns, was associated with a lower risk of anxiety. Engagement in recreational MVPA is significantly associated with reduced anxiety risk, with no substantial differences among the three PA patterns. The results of the sensitivity analysis confirmed the robustness of our findings. Subgroup analysis revealed a significant association between the WW pattern and lower risk of anxiety in middle- and low-income populations, while this association was not significant in high-income groups, suggesting that the WW pattern may serve as a therapeutic approach for alleviating anxiety in economically disadvantaged regions. Additionally, the WW pattern was linked to a lower risk of anxiety among individuals with diabetes, though the association in non-diabetic populations suggested a trend toward significance. Factors such as gender, age, race, education level, marital status, BMI, smoking status, alcohol consumption, sleep duration, and hypertension did not significantly influence the relationship between the WW pattern and risk of anxiety. The results of RCS analysis indicated that a significantly nonlinear relationship existed between weekly recreational MVPA and the risk of anxiety.
Currently, limited evidence exists on the relationship between the WW pattern and anxiety. A recent UK-based study investigating the relationship between the WW pattern and brain health found that both individuals engaging in concentrated weekend MVPA and those maintaining regular exercise exhibited a lower risk of anxiety compared to inactive individuals [28]. Additionally, a longitudinal study of Chinese adolescents (aged 9–19) found that as they aged, weekday MVPA decreased while weekend and holiday light activity increased. Notably, weekend MVPA was associated with lower anxiety levels after six months [29]. Furthermore, another study utilizing a rat model of chronic mild stress to examine depressive-like behaviors and cognitive impairments found that the WW pattern was equally effective as the continuous exercise pattern in reducing anxiety behaviors, enhancing cognitive function, and alleviating depressive-like symptoms [30]. While existing research highlights the benefits of the WW pattern, our study supports these findings and extends them in key areas. First, we utilized large-scale data from a U.S. population, which had not been previously examined. Our study included participants aged over 20, providing a more realistic reflection of the daily work-life rhythm in the adult population. Second, our study offered a more comprehensive subgroup analysis compared to the UK-based study, which was limited to gender, age, and race [28]. We extended our analysis to include socioeconomic status and health conditions, uncovering significant variations in the association between the WW pattern and risk of anxiety across different subgroups. These findings provided valuable insights into how the WW pattern may benefit specific populations, particularly those with lower socioeconomic status or chronic conditions such as diabetes. Third, while most studies classified PA patterns into three categories: inactive (less than 150 min of MVPA per week), WW, and regularly active, our study introduced a more nuanced categorization by dividing PA into four patterns: inactive, insufficiently active, WW, and regularly active. This approach provides new insights and reveals that even when recreational MVPA does not meet the weekly recommended threshold, it is still significantly associated with lower risk of anxiety.
There appears to be a potential dose-response relationship between PA and anxiety [31]. While most studies have focused on the total volume and intensity of PA [32–38], few have explored the role of exercise frequency. A meta-analysis of 49 studies found that exercising 3–4 times a week significantly improved anxiety symptoms while exercising either 1–2 times or more than 5 times per week showed only slight improvements without statistical significance [14]. However, another updated meta-analysis reported no significant differences in anxiety improvements across exercise frequencies ranging from 1 to 5 times per week [39]. These mixed findings suggested that the effect of frequency on anxiety might be less critical than previously thought. Our study supported this idea by highlighting the WW pattern, in which individuals engage in MVPA at lower frequencies but meet the recommended weekly PA volume. We found that the WW pattern is also associated with a reduced risk of anxiety, suggesting that the total volume of PA, rather than frequency, maybe the key determinant in reducing anxiety risk.
The mechanisms underlying the relationship between exercise and anxiety involve multiple neurobiological processes. Firstly, exercise mitigates anxiety by reducing the S-nitrosylation of gephyrin protein in the basolateral amygdala, enhancing GABAergic neurotransmission and alleviating anxiety symptoms [40]. Secondly, exercise-induced lactylation boosts the lactylation of cortical synaptic proteins, particularly synaptosome-associated protein 91, which supports synaptic integrity and promotes stress resilience [41]. Additionally, exercise modulates noradrenergic and galaninergic systems, especially under stress conditions, further influencing anxiety-related behaviors [42]. Broman-Fulks et al. (2018) also suggest that anxiety sensitivity mediates the relationship between exercise frequency and anxiety and depression symptoms [43]. Lastly, the medial prefrontal cortex to basolateral amygdala circuit plays a pivotal role in these anxiolytic effects, with exercise training strengthening this pathway to enhance resilience against environmental stress [44].
The primary strength of this study lies in the use of nationally representative data from the NHANES database, along with a detailed classification of PA patterns. Our findings demonstrate that even recreational PA below the recommended threshold is significantly associated with reduced anxiety risk, which has not been observed previously, offering new insights for public health policy. Additionally, comprehensive subgroup analyses reveal that the WW pattern may have therapeutic potential for alleviating anxiety, particularly among individuals with lower or middle-income levels and patients with diabetes. Some limitations exist in this research. Due to the cross-sectional design, causal relationships between WW and anxiety cannot be established. Furthermore, a limitation of this study is the use of self-reported physical activity data, which may be subject to recall and social desirability biases. Additionally, the data from multiple NHANES cycles (2007–2008, 2009–2010, and 2011–2012) could introduce measurement variability. The lack of distinction between different anxiety subtypes and the exclusion of occupational PA may also limit the comprehensiveness of our findings. Although we adjusted for multiple confounders, some variables might still influence the observed association. Future studies utilizing longitudinal designs are warranted to investigate the long-term effects of WW and to further validate the effectiveness of WW within diverse populations. Considering that research has already shown that exercise can reduce stress and promote the release of endorphins [45–47], future research should explore the underlying mechanisms through which the WW pattern exerts a protective effect on anxiety, as well as investigate the causal relationship between the WW pattern and anxiety. Lastly, in our sensitivity analysis, when depression was included as a covariate, the association between the WW pattern and anxiety was no longer statistically significant in Model 3. However, this association remained significant in the unweighted and IPTW models. This discrepancy may be attributed to the high collinearity between depression and anxiety, as well as the impact of weighting methods on statistical power, highlighting the need for further validation of our findings.
Conclusion
In conclusion, the weekend warrior and regularly active patterns were all associated with similar reductions in the risk of anxiety among adult Americans. These findings suggest that for individuals unable to maintain consistent PA throughout the week, adopting a WW pattern can be an effective alternative to mitigate anxiety. Promoting flexible PA patterns that align with diverse lifestyles is essential, especially for those facing time constraints or other barriers to regular weekday exercise.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the staff of the National Health and Nutrition Examination Survey program for their effort in data collection.
Abbreviations
- WW
Weekend warrior
- NHANES
National Health and Nutrition Examination Survey
- PA
Physical activity
- MVPA
Moderate to vigorous intensity physical activity
- BMI
Body mass index
- PIR
Poverty income ratio
- FPG
Fasting plasma glucose
- HbA1c
Glycol hemoglobin
- SD
Standard deviation
- ORs
Odds ratios
- CI
Confidence intervals
- RCS
Restricted cubic splines
- IPTW
Inverse probability treatment weighting
Author contributions
ZH. C and JY. T contributed to the conception and design, acquisition, analysis, interpretation of the data, and drafting of the manuscript or critical revision for important intellectual content. JH. X and JQ. J completed the software analysis and data visualization. XM. L contributed to the conception and design and reviewing of the manuscript or critical revision for important intellectual content. All authors approved the final version and agree to be accountable for all aspects of the work.
Funding
Not applicable.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
All study participants provided informed consent and the study protocol was approved by the Ethics Review Board of the National Center for Health Statistics (NCHS). Information can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/participant.htm).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kogan CS, Stein DJ, Maj M, First MB, Emmelkamp PM, Reed GM. The classification of anxiety and fear-related disorders in the ICD-11. Depress Anxiety. 2016;33(12):1141–54. [DOI] [PubMed] [Google Scholar]
- 2.Remes O, Brayne C, van der Linde R, Lafortune L. A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav. 2016;6(7):e00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45(8):1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilmer MT, Anderson K, Reynolds M. Correlates of quality of life in anxiety disorders: review of recent research. Curr Psychiatry Rep. 2021;23(11):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JRT, den Boer JA, Fineberg NA, Knapp M, Scott J, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567–96. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin DS, Waldman S, Allgulander C. Evidence-based pharmacological treatment of generalized anxiety disorder. Int J Neuropsychopharmacol. 2011;14(05):697–710. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Goicoechea C, Heshmati S, Carpenter JK, Hofmann SG. Efficacy of cognitive behavioral therapy for anxiety-related disorders: a Meta-analysis of recent literature. Curr Psychiatry Rep. 2022;25(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo‐controlled trials. Depress Anxiety. 2018;35(6):502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell LM, Joshi U, Patel V, Lu C, Naslund JA. Economic evaluations of internet-based psychological interventions for anxiety disorders and Depression: a systematic review. J Affect Disord. 2021;284:157–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh B, Olds T, Curtis R, Dumuid D, Virgara R, Watson A, Szeto K, O’Connor E, Ferguson T, Eglitis E, et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: an overview of systematic reviews. Br J Sports Med. 2023;57(18):1203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeon G, Curtis J, Rosenbaum S. Promoting physical activity for mental health: an updated evidence review and practical guide. Curr Opin Psychiatry. 2022;35(4):270–6. [DOI] [PubMed] [Google Scholar]
- 12.Paluska SA, Schwenk TL. Physical activity and Mental Health. Sports Med. 2000;29(3):167–80. [DOI] [PubMed] [Google Scholar]
- 13.Mochcovitch MD, Deslandes AC, Freire RC, Garcia RF, Nardi AE. The effects of regular physical activity on anxiety symptoms in healthy older adults: a systematic review. Revista Brasileira De Psiquiatria. 2016;38(3):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wipfli BM, Rethorst CD, Landers DM. The Anxiolytic effects of Exercise: a Meta-analysis of randomized trials and dose–response analysis. J Sport Exerc Psychol. 2008;30(4):392–410. [DOI] [PubMed] [Google Scholar]
- 15.Lee IM. The Weekend Warrior and Risk of Mortality. Am J Epidemiol. 2004;160(7):636–41. [DOI] [PubMed] [Google Scholar]
- 16.Khurshid S, Al-Alusi MA, Churchill TW, Guseh JS, Ellinor PT. Accelerometer-derived Weekend Warrior Physical Activity and Incident Cardiovascular Disease. JAMA. 2023;330(3):247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donovan G, Petermann-Rocha F, Ferrari G, Lee IM, Hamer M, Stamatakis E, Sarmiento OL, Ibáñez A, Lopez-Jaramillo P. Associations of the ‘weekend warrior’ physical activity pattern with all-cause, cardiovascular disease and cancer mortality: the Mexico City prospective study. Br J Sports Med. 2024;58(7):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donovan G, Lee IM, Hamer M, Stamatakis E. Association of Weekend Warrior and other Leisure Time physical activity patterns with risks for All-Cause, Cardiovascular Disease, and Cancer Mortality. JAMA Intern Med. 2017;177(3):335–42. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Xia F, Li Q, Luo X, Wu J. The associations of Weekend Warrior activity patterns with the visceral Adiposity Index in US adults: repeated cross-sectional study. JMIR Public Health Surveillance. 2023;9(1):e41973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R, Wang K, Chen Q, Zhang M, Yang H, Zhang M, Qi K, Zheng M, Wang Y, He Q. Weekend warrior physical activity pattern is associated with lower depression risk: findings from NHANES 2007–2018. Gen Hosp Psychiatry. 2023;84:165–71. [DOI] [PubMed] [Google Scholar]
- 21.Almohamad M, Kaye EK, Mofleh D, Spartano NL. The association of sedentary behaviour and physical activity with periodontal disease in NHANES 2011–2012. J Clin Periodontol. 2022;49(8):758–67. [DOI] [PubMed] [Google Scholar]
- 22.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for americans. JAMA. 2018;320(19):2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Yang W, Liu F. The associations of weekend warrior and other physical activity patterns with the risk of all-cause and cardiovascular disease mortality in people with diabetes mellitus and chronic kidney disease: from NHANES 2007–2020. Int Urol Nephrol. 2023;56(5):1703–12. [DOI] [PubMed] [Google Scholar]
- 24.Gui J, Ding R, Huang D, Wang L, Han Z, Yang X, Yang J, Luo H, Jiang L. Associations between urinary heavy metals and anxiety among adults in the National Health and Nutrition Examination Survey (NHANES), 2007–2012. Chemosphere. 2023;341:140085. [DOI] [PubMed] [Google Scholar]
- 25.Wen Z, Bai L, Wu S, Chen J, Jama HA, Sawmadal JD. Association of serum vitamin D with anxiety in US adults: a cross-sectional study. Front Nutr. 2024;11:1371170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dantzer JA, Keet CA. Anxiety associated with food allergy in adults and adolescents: an analysis of data from the National Health and Nutrition Examination Survey (NHANES) 2007–2010. J Allergy Clin Immunol Pract. 2020;8(5):1743–e17461745. [DOI] [PubMed] [Google Scholar]
- 27.Andresen EM, Catlin TK, Wyrwich KW, Jackson-Thompson J. Retest reliability of surveillance questions on health related quality of life. J Epidemiol Community Health. 2003;57(5):339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min J, Cao Z, Duan T, Wang Y, Xu C. Accelerometer-derived ‘weekend warrior’ physical activity pattern and brain health. Nat Aging 2024. [DOI] [PubMed]
- 29.Yan W, Wang Y, Yuan Y, Farid M, Zhang P, Peng K. Timing matters: a longitudinal study examining the effects of physical activity intensity and timing on adolescents’ Mental Health outcomes. J Youth Adolesc. 2024;53(10):2320–31. [DOI] [PubMed] [Google Scholar]
- 30.Öztürk ÇÇ, Ataoğlu SN, Arvas A, Tokol H, Yaprak H, Gürel S, Levent HN, Akakın D, Şahin A, Çakır B, et al. Weekend warrior exercise model for protection from chronic mild stress–induced depression and ongoing cognitive impairment. Acta Neurobiol Exp. 2023;83(1):10–24. [DOI] [PubMed] [Google Scholar]
- 31.Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6 Suppl):S587–597. discussion 609– 510. [DOI] [PubMed] [Google Scholar]
- 32.Goldfield GS, Henderson K, Buchholz A, Obeid N, Nguyen H, Flament MF. Physical activity and psychological adjustment in adolescents. J Phys Act Health. 2011;8(2):157–63. [DOI] [PubMed] [Google Scholar]
- 33.Heesch KC, Burton NW, Brown WJ. Concurrent and prospective associations between physical activity, walking and mental health in older women. J Epidemiol Community Health. 2011;65(9):807–13. [DOI] [PubMed] [Google Scholar]
- 34.Jussila JJ, Pulakka A, Ervasti J, Halonen JI, Mikkonen S, Allaouat S, Salo P, Lanki T. Associations of leisure-time physical activity and active school transport with mental health outcomes: a population-based study. Scand J Med Sci Sports. 2023;33(5):670–81. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, Jeon SW, Lee MY, Shin DW, Lim WJ, Shin YC, Oh KS. The Association between physical activity and anxiety symptoms for General Adult populations: an analysis of the dose-response relationship. Psychiatry Investig. 2020;17(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDowell CP, Gordon BR, Andrews KL, MacDonncha C, Herring MP. Associations of physical activity with anxiety symptoms and status: results from the Irish longitudinal study on ageing. Epidemiol Psychiatr Sci. 2019;28(4):436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimura A, Masuya J, Yokoi K, Morishita C, Kikkawa M, Nakajima K, Chen C, Nakagawa S, Inoue T. Too much is too little: estimating the optimal physical activity level for a healthy mental state. Front Psychol. 2022;13:1044988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smout S, Gardner LA, Newton N, Champion KE. Dose-response associations between modifiable lifestyle behaviours and anxiety, depression and psychological distress symptoms in early adolescence. Aust N Z J Public Health. 2023;47(1):100010. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Sanchez CP, Schuch FB, Seedat S, Louw QA, Stubbs B, Rosenbaum S, Firth J, van Winkel R, Vancampfort D. The anxiolytic effects of exercise for people with anxiety and related disorders: an update of the available meta-analytic evidence. Psychiatry Res. 2021;302:114046. [DOI] [PubMed] [Google Scholar]
- 40.Yang PF, Nie TL, Sun XN, Xu LX, Ma C, Wang F, Long LH, Chen JG. Wheel-running Exercise alleviates anxiety‐like Behavior via Down‐Regulating S‐Nitrosylation of Gephyrin in the basolateral amygdala of male rats. Adv Sci. 2024;11(34):e2400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan L, Wang Y, Hu H, Yang D, Wang W, Luo Z, Wang Y, Yang F, So K-F, Zhang L. Physical exercise mediates cortical synaptic protein lactylation to improve stress resilience. Cell Metabol. 2024;36(9):2104–e21172104. [DOI] [PubMed] [Google Scholar]
- 42.Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehavioral Reviews. 2012;36(9):1965–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broman-Fulks JJ, Abraham CM, Thomas K, Canu WH, Nieman DC. Anxiety sensitivity mediates the relationship between exercise frequency and anxiety and depression symptomology. Stress Health. 2018;34(4):500–8. [DOI] [PubMed] [Google Scholar]
- 44.Luo Z, Chen J, Dai Y, So K-F, Zhang L. Treadmill exercise modulates the medial prefrontal-amygdala neural circuit to improve the resilience against chronic restraint stress. Commun Biology. 2023;6(1):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerra G, Volpi R, Delsignore R, Caccavari R, Gaggiotti MT, Montani G, Maninetti L, Chiodera P, Coiro V. ACTH and beta-endorphin responses to physical exercise in adolescent women tested for anxiety and frustration. Psychiatry Res. 1992;41(2):179–86. [DOI] [PubMed] [Google Scholar]
- 46.Zieff GH, Stoner L, Frank B, Gaylord S, Battle S, Hackney AC. Aerobic exercise, mindfulness meditation, and stress-reduction in high-stress, college-based young adults: a pilot study. J Am Coll Health. 2024;72(5):1331–5. [DOI] [PubMed] [Google Scholar]
- 47.Lee YH, Kim H, Hwang J, Noh S. Effectiveness of Mobile-Based Progressive and fixed physical activity on Depression, stress, anxiety, and quality of life outcomes among adults in South Korea: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2024;12:e55578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.