Abstract
Objective
Assess the effect of fear of cancer recurrence (FCR) and coping strategies on patients with oral cancer’s post-traumatic growth (PTG).
Methods
A total of 255 patients with oral cancer participated and were investigated using the Chinese version of the Posttraumatic Growth Inventory (PTGI), the Fear of Progression Questionnaire-Short Form (FOP-Q-SF), and the Simplified Coping Style Questionnaire (SCSQ) in this cross-sectional study. Descriptive, univariate, Pearson correlation analyses, and multiple linear regression analyses were performed.
Results
Higher FCR scores were negatively associated with PTG (r = -0.646). Negative coping strategies were negatively correlated with PTG (r = -0.219). Positive coping strategies positively correlated with PTG (r = 0.482). Regression analysis indicated that the treatment of surgery combined with chemotherapy and radiotherapy, FCR, and coping strategies had significant independent influences on patients with oral cancer’s PTG.
Conclusions
Reducing fear of recurrence and improving one’s capacity for proactive coping in the face of traumatic situations are necessary for post-traumatic growth stimulation. Thus, it is important to develop and implement focused therapies aimed at enhancing post-traumatic growth in patients with oral cancer.
Keywords: Coping strategies, Fear of cancer recurrence, Oral cancer, PTG, Post-traumatic growth
Background
One of the most prevalent malignant tumors in the head and neck region is oral cancer, which includes malignancies of various regions of the mouth and throat [1]. As per the most recent statistics released by the International Agency for Research on Cancer in 2020 [2], oral cancer is the 15th largest cause of mortality globally and ranks 16th among malignant tumors. The National Cancer Center has released the latest cancer statistics, stating that the number of cases of oral cancer in China reaches 52,200 annually. In recent years, the burden of oral cancer in China has been increasing [3]. Patients suffer from severe psychological suffering in addition to bodily harm as a result of the illness and its treatment. Research in the field of psycho-oncology has indicated that positive psychosocial elements are vital to cancer patients’ ability to heal [4]. It is possible to view having cancer and receiving treatment as traumatic experiences that can result in traumatic reactions [5]. Both unpleasant feelings and chances for personal growth can result from traumatic experiences [6]. Indeed, a growing body of research is dedicated to exploring positive psychological changes in patients during the course of their illness as positive psychology advances, and several survivors have shared positive developments and personal growth [7, 8].
As a part of positive psychology, post-traumatic growth (PTG) refers to the positive psychological changes experienced by individuals during their struggle with traumatic life events and difficulties, including a greater appreciation of life, improved interpersonal relationships, higher spiritual development, increased possibilities in life, and enhancement of the personal strengths [5, 9]. Studies that are cross-sectional and longitudinal show a positive correlation between PTG and health-related quality of life [10–13]. PTG can encourage healthy habits, improve physical and mental health, and lessen psychological discomfort, according to a systematic review [14]. In addition, research has demonstrated that positive psychology can enhance general quality of life and assist in managing symptoms [15]. An increasing number of studies have shown that many cancer survivors experience PTG [16], such as patients with breast cancer, lung cancer, and so on. And PTG has been reported in patients with oral cancer and is garnering interest [17, 18]. They are more likely to pay more attention to their physical and mental well-being and enhance their prognosis better. However, PTG may not always arise immediately from a traumatic experience because there are a number of variables that can help or impede PTG’s development as the recovery process. Thus, it is crucial to assess the PTG level of patients with oral cancer and investigate the factors that influence PTG.
There is a relationship between PTG and fear of cancer recurrence (FCR), according to a cross-sectional study involving 190 patients with head and neck cancer [19]. FCR refers to the fear and worry of cancer returning or recurring [20]. For cancer patients, FCR is the primary cause of psychological discomfort [21]. Additionally, FCR has also been reported in patients with oral cancer [22, 23]. Patients with low levels of FCR typically exhibit high degrees of optimism and well-being, which may enhance their quality of life and promote their PTG [19]. Therefore, the detrimental impact of FCR on PTG may be useful for cancer patients.
According to recent literature reviews, coping strategies may be crucial for PTG [24, 25]. Coping strategies refers to the cognitive or behavioral regulatory strategies that individuals employ in the face of various stressors, generally classified as positive or negative [26]. There are several different coping strategies, such as cognitive coping, problem-centered, and emotion-centered coping [27]. Patients’ coping strategies play a crucial role, as higher levels of positive coping, such as looking for social assistance, can predict better health-related quality of life and lower levels of depression and anxiety. Elevated negative coping strategies, such as escape from reality, result in worse health-related quality of life along with increased depression and anxiety [28].
The stress-coping theory [29] suggests that the outcomes that individuals experience when exposed to stressors primarily depend on two crucial psychological processes: cognitive assessment and coping. FCR can be regarded as a cognitive assessment, and receiving a cancer diagnosis might be regarded as a traumatic stressor. According to the stress-coping theory, the growth level of patients with oral cancer after trauma may be affected by FCR and coping strategies.
PTG is a significant psychological improvement that should be studied more in individuals with oral cancer. Patients with oral cancer have not been the topic of many studies, despite some prior research on PTG. Patients with oral cancer may experience different effects on their PTG levels from FCR and coping strategies. Nevertheless, no study to date has focused on the assessment of the connection between these psychological variables in patients with oral cancer. This study aimed to comprehend the profiles and ascertain the relationship between FCR, coping strategies, and the level of PTG. The findings might be useful for healthcare professionals to develop a prevention and intervention conceptual reference framework to assist patients with oral cancer in managing PTG. This study proposes the following two hypotheses based on stress-coping theory:
H1. FCR is negatively associated with PTG.
H2. Positive coping strategies are positively correlated with PTG; negative coping strategies are negatively associated with PTG.
Methods
Aim
The study aims to investigate the effect of FCR and coping strategies on patients with oral cancer’s PTG.
Design
With the use of a questionnaire survey, this study was a cross-sectional study.
Study settings and participants
This cross-sectional study was carried out at the inpatient department of three hospitals in Chongqing, China, from September 2023 to April 2024. This study was approved by the Ethics Committee of the Stomatological Hospital of Chongqing Medical University (no. 2023/154). The participants were recruited using a convenience sampling method. The inclusion criteria were as follows: diagnosis of oral cancer based on histopathology or cytology; they were adult patients older than 18 years; those without a prior history of mental disorders; and those who were physically able to complete questionnaires. Before being invited to join, those who satisfy all eligibility requirements will get information on the study goals, methods, guarantees of anonymity, and their ability to withdraw from the study.
Measures
Clinical and sociodemographic characteristics
After studying the literature and conducting study group discussions, researchers construct questionnaires to collect clinical and sociodemographic data from participants. Sociodemographic data included gender, age, marital status, habitation, employment, education, monthly family income, and religious beliefs. Clinical data included cancer staging, treatment, and time since diagnosis. Sociodemographic information was self-reported. Clinical data were provided by electronic patient records.
Outcome variable (post-traumatic growth)
The Post-traumatic Growth Inventory (PTGI) [30] was used to evaluate the level of post-traumatic growth among the participants. The Chinese Posttraumatic Growth Inventory (C-PTGI) is validated by Wang and colleagues. The C-PTGI is a self-reported instrument consisting of 20 items designated into 5 domains, including new possibilities in life, relationships with others, spiritual change, personal strength, and appreciation of life. Items were rated on a 6-point Likert Scale (0 = not at all, 5 = very much indeed). The total score ranges from 0 to 100. The higher the PTGI score, the higher the level of PTG. The PTGI demonstrates good internal consistency. In the present study, the reliability coefficients of the five subscales (spiritual change, appreciation of life, relationships with others, new possibilities in life, and personal strength) of PTGI were 0.893, 0.909, 0.852, 0.882, and 0.849, respectively.
Explanatory variables
Fear of cancer recurrence: The level of fear of cancer recurrence was measured by the Fear of Progression Questionnaire-Short Form (FOP-Q-SF). This questionnaire consists of 12 items across two dimensions (physical health, social and family). This instrument uses a Likert Scale format ranging from 1 (never) to 5 (very often), and its total score ranges from 12 to 60. The FOP-Q-SF have been reported with excellent internal consistency.
Coping strategies: To assess participants’ coping strategies, we use the Simplified Coping Style Questionnaire (SCSQ), developed by Chinese scholar Xie Yaning [31], as an assessment tool. The 20-item instrument uses a Likert scale format with both positively and negatively worded items. The positive coping subscale has twelve items, while the negative coping subscale has eight. The range of scores for the positive and negative coping dimensions is 0–36 and 0–24, respectively. The higher score of each subscale presents the more frequent usage of that coping style. The good reliability and construct validity have been confirmed in the Chinese population [32].
Data collection
To ensure the consistency of questionnaire collection, the researcher conducted unified training. During the survey, the researchers gave the patients an explanation of the survey’s goal and the essentials of filling out the pertinent scales, and they got their permission. Patients were advised to give honest answers and independently finish the questionnaire. Researchers should provide explanations for any questions that came up throughout the patient’s response. If any responses are missing from the questionnaires, the researcher will inform the patient to fill out questionnaires completely. The questionnaire took 15 to 20 min to complete. The period of data collection was September 2023–April 2024. 268 questionnaires were distributed in all, and 255 of them were returned after being filled out anonymously.
Data analysis
Frequencies and percentages were employed to describe the sociodemographic data; Displaying continuous variables using mean and standard deviation (SD); The independent-sample t-test or analysis of variance (ANOVA) was used to compare the scores of PTG, FCR, and coping strategies among patients with oral cancer and sociodemographic characteristics. The association between FCR, coping strategies, and PTG was tested using the Pearson correlation coefficient analysis (r). Multiple linear regression was used to analyze the factors influencing patients with oral cancer’s PTG. The data were analyzed using IBM SPSS Statistics software version 27.0. The significance level was set as 0.05 (two-tailed).
Results
Sociodemographic and clinical characteristics of the study sample
Participants were within the age group of 27–85 years with a mean score of 61.07 ± 11.58. The participants were mostly men (55.69%, n = 142), married (74.90%, n = 191), and resided in cities or towns (66.67%, n = 170). Most patients were diagnosed with stage II cancer (34.12%, n = 87). Most patients do not experience cancer recurrence (81.57%, n = 208). Other general characteristics about the participants are presented in Table 1.
Table 1.
Demographic characteristics of the patients with oral cancer and univariate analysis of patients with oral Cancer’s PTG (n = 255)
| Variable | Items | Frequency (%) | PTGI score (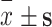 ) ) |
t/F | p |
|---|---|---|---|---|---|
| Gender | Male | 142(55.69%) | 56.68 ± 18.92 | -0.187 | 0.852 |
| Female | 113(44.31%) | 57.12 ± 18.47 | |||
| Age (years) | < 40 | 9(3.5%) | 53.33 ± 23.23 | 0.881 | 0.451 |
| 40 ~ 59 | 96(37.6%) | 55.20 ± 18.17 | |||
| 60 ~ 79 | 144(56.5%) | 58.45 ± 18.64 | |||
| ≥ 80 | 6(2.4%) | 51.33 ± 22.05 | |||
| Marital status | Married | 191(74.90%) | 58.32 ± 18.45 | 1.724 | 0.163 |
| Unmarried | 18(7.06%) | 50.89 ± 18.43 | |||
| Divorced | 21(8.24%) | 52.38 ± 20.28 | |||
| Widowed | 25(9.80%) | 53.48 ± 18.54 | |||
| Occupation | Unemployed/Retired | 188(73.73%) | 58.20 ± 18.57 | -1.900 | 0.060 |
| Incumbency | 67(26.27%) | 53.14 ± 18.66 | |||
| Habitation | City/town | 170(66.67%) | 58.79 ± 18.13 | 2.335 | 0.020* |
| Countryside | 85(33.33%) | 53.05 ± 19.30 | |||
| Education | Primary school and below | 68(26.67%) | 58.84 ± 19.30 | 3.202 | 0.024* |
| Junior high school | 75(29.41%) | 54.43 ± 19.24 | |||
| High/polytechnic school | 79(30.98%) | 58.60 ± 18.97 | |||
| Junior college and above | 33(12.94%) | 64.58 ± 12.52 | |||
| Family monthly income (Chinese yuan/RMP) | ≤ 2000 | 45(17.65%) | 50.93 ± 18.66 | 2.863 | 0.037* |
| 2001 ~ 5000 | 79(30.98%) | 57.83 ± 19.38 | |||
| 5001 ~ 10,000 | 88(34.51%) | 56.42 ± 18.99 | |||
| >10,000 | 43(16.86%) | 62.28 ± 15.29 | |||
| Religious beliefs | no | 241(94.51%) | 57.00 ± 18.80 | -0.445 | 0.657 |
| yes | 14(5.49%) | 54.71 ± 17.08 | |||
| TNM stage | I | 67(26.27%) | 63.04 ± 15.21 | 3.967 | 0.009* |
| II | 87(34.12%) | 55.86 ± 19.54 | |||
| III | 57(22.35%) | 55.26 ± 19.62 | |||
| IV | 44(17.25%) | 51.59 ± 18.70 | |||
| Recurrence | no | 208(81.57%) | 58.25 ± 18.01 | -2.302 | 0.025* |
| yes | 47(18.43% | 50.79 ± 20.52 | |||
| Treatment modality | Surgery | 156(61.18%) | 59.84 ± 17.98 | 9.315 | < 0.001* |
| Surgery+radiotherapy | 22(8.63%) | 59.27 ± 18.42 | |||
| Surgery+chemotherapy | 28(10.98%) | 58.64 ± 18.69 | |||
| Surgery+radiotherapy+chemotherapy | 39(15.29%) | 41.43 ± 14.43 | |||
| Chemotherapy | 10(3.92) | 61.90 ± 17.00 | |||
| Time since cancer diagnosis | <1month | 104(40.78%) | 57.89 ± 18.82 | 1.059 | 0.367 |
| 1month ~ 6months | 69(27.06%) | 57.09 ± 18.66 | |||
| 6months ~ 1year | 50(19.61%) | 52.88 ± 18.87 | |||
| >1year | 32(12.55%) | 59.38 ± 17.95 |
*p < 0.05
Independent-sample t tests showed significant differences in patients with oral cancer’s PTG by habitation (t = 2.335, P = 0.020) and recurrence (t = -2.302, P = 0.025). One-way analysis of variance showed significant differences in patients with oral cancer’s PTG by their education (F = 3.202, P = 0.024), monthly family income (F = 2.863, P = 0.037), cancer staging (F = 3.967, P = 0.009), and treatment (F = 9.315, P < 0.001) (shown in Table 1).
Descriptive and correlational analyses of the main variables
As indicated in Table 2, the mean values of PTG, FCR, dimension of positive coping strategies, and dimension of negative coping strategies were 56.88 ± 18.69, 32.55 ± 9.77, 19.86 ± 9.82, and 8.72 ± 6.76, respectively. Pearson correlation testing showed that PTG had a significant positive correlation with positive coping strategies (r = 0.482). PTG had a significant negative correlation with FCR (r = -0.646) and negative coping strategies (r = -0.219) (shown in Table 3).
Table 2.
Mean scores of variables (n = 255)
| Variables | Number of entries | Min | Max | Average total score ( ) ) |
Average item score (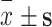 ) ) |
|---|---|---|---|---|---|
| PTGI | 20 | 17 | 85 | 56.88 ± 18.69 | 2.86 ± 0.94 |
| FOP-Q-SF | 12 | 17 | 56 | 32.55 ± 9.77 | 2.71 ± 0.81 |
| Subscale of positive coping strategies | 12 | 2 | 35 | 19.86 ± 9.82 | 1.66 ± 0.82 |
| Subscale of negative coping strategies | 8 | 1 | 24 | 8.72 ± 6.76 | 1.09 ± 0.85 |
Table 3.
Correlations between FCR, coping strategies and PTG (n = 255)
| Variable | PTG | FCR | Positive coping strategies | Negative coping strategies |
|---|---|---|---|---|
| PTG | 1 | — | — | — |
| FCR | -0.646* | 1 | — | — |
| Positive coping strategies | 0.482* | -0.455* | 1 | — |
| Negative coping strategies | -0.219* | 0.199* | 0.104 | 1 |
*p < 0.05
Regression analyses
A multivariate linear regression analysis was performed with all statistically significant factors (habitation, education, family monthly income, TNM stage, recurrence, treatment modality) determined using the t-test and ANOVA, with FCR and coping strategies as independent variables, and PTG as the dependent variable. The multiple linear regression results are presented in Table 4. The results indicated that the treatment of surgery combined with chemotherapy and radiotherapy, FCR, and coping strategies all had significant independent influences on patients with oral cancer’s PTG. They contributed to explaining 49.1% of the variance in PTG.
Table 4.
Multiple linear regression analysis of correlating factors of PTG of patients with oral cancer (n = 255)
| Independent variable | Unstandardized coefficient | Standardized coefficient | ||||
|---|---|---|---|---|---|---|
| B | Std. error | β | t | p | VIF | |
| (Constant) | 84.598 | 7.602 | 11.129 | < 0.001* | ||
| Habitation | -1.078 | 2.389 | -0.027 | -1.113 | 0.652 | 1.810 |
| Education | 0.617 | 1.059 | 0.033 | 0.583 | 0.561 | 1.604 |
| Family monthly income (Chinese yuan/RMP) | -0.200 | 1.298 | -0.010 | -0.154 | 0.878 | 2.264 |
| TNM stage | -0.985 | 0.885 | -0.055 | -1.113 | 0.267 | 1.206 |
| Recurrence | 1.535 | 2.648 | 0.032 | 0.580 | 0.563 | 1.506 |
| Surgery+radiotherapy | 0.933 | 3.128 | 0.014 | 0.298 | 0.766 | 1.103 |
| Surgery+chemotherapy | -0.475 | 2.850 | -0.008 | -0.167 | 0.868 | 1.135 |
| Chemotherapy | -0.008 | 4.400 | < 0.001 | -0.002 | 0.998 | 1.043 |
| The treatment of surgery combined with chemotherapy and radiotherapy | -9.301 | 2.671 | -0.179 | -3.482 | 0.001* | 1.320 |
| Time since cancer diagnosis | -0.198 | 0.954 | -0.011 | -0.207 | 0.836 | 1.431 |
| FCR | -0.896 | 0.103 | -0.467 | -8.725 | < 0.001* | 1.424 |
| Dimension of positive coping strategies | 0.434 | 0.104 | 0.228 | 4.178 | < 0.001* | 1.477 |
| Dimension of negative coping strategies | -0.331 | 0.132 | -0.119 | -2.498 | 0.013* | 1.135 |
Note: F = 19.777, * p < 0.05, R2 = 0.491(adjusted)
Abbreviations: B, unstandardized regression coefficient; β, standardized regression coefficient; VIF, variance inflation factor
Discussion
This work focused on exploring the current situation and relation among PTG, FCR, and coping strategies of patients with oral cancer. The results showed that the treatment of surgery combined with chemotherapy and radiotherapy, FCR, and coping strategies all had significant independent influences on patients with oral cancer’s PTG. Furthermore, PTG is negatively associated with FCR and negative coping strategies but positively correlated with positive coping strategies. Therefore, this work provides theoretical reference for healthcare professionals to take effective measures targeting the improvement of PTG, which can finally benefit patients’ medical outcomes and health well-beings.
The post-traumatic growth level of patients with oral cancer needs to be improved
Our study revealed that the PTG level of patients with oral cancer is moderate, with a score of 56.88 ± 18.69, which is consistent with the research results of Zhu [33] on Chinese gastric cancer patients and Sharp [34] on patients with head and neck cancer. Show that patients can still promote positive psychological change in spite of their disease. According to the results of regression analyses, patients who underwent a comprehensive treatment that included surgery, radiotherapy, and chemotherapy experienced less PTG than those who only got surgery. Studies have shown that patients with more complex treatment methods and longer treatment cycles suffer more psychological, physical, and economic burdens compared to cancer patients with a single treatment method [35]. Comprehensive treatment frequently results in more severe side effects that not only harm the patient’s physical health but also increase their psychological stress. Consequently, patients struggle to keep a positive mental attitude, which hinders their psychological development. There is still room for improvement in the PTG levels of patients with oral cancer. Therefore, medical professionals should place a high value on stimulating patients’ internal positive psychological factors and helping them in achieving personal growth in the face of disease.
The relationship between post-traumatic growth and fear of cancer recurrence
Our research reveals a significant negative correlation between FCR and PTG. This means that our research hypothesis H1 (FCR is negatively associated with PTG) has been accepted. This demonstrates FCR’s great predictive power in forming PTG, and in order to stimulate PTG in patients, uncertainty about the disease must be reduced, as well as concerns and fears about cancer recurrence. Our findings about the correlation between PTG and FCR are consistent with Nik’s research on patients with head and neck cancer [19]. The disease itself and various treatments can damage the patient’s oral function [36], such as decreased motor function for swallowing and chewing, decreased language function for pronunciation and enunciation, and changes in facial appearance, which can lead to negative emotional experiences such as anxiety, depression, and fear of recurrence in patients [37]. The negative emotions can activate the sympathetic nervous system and the hypothalamus-pituitary-adrenal system, as well as trigger the release of stress hormones such as glucocorticoids and catecholamines [38]. According to research, stress hormones were mostly responsible for surgical adverse reactions, which made patients’ symptoms worse and made their PTG less effective [39]. This set of statements provides support for the results of our study by revealing the connection between negative emotions, stress hormones, and PTG, which explains why FCR and PTG are negatively connected. Positive emotions have been demonstrated in numerous studies to stimulate PTG [40, 41], suggesting that altering an individual’s cognitive functioning and activating internal resources can facilitate PTG. In addition, the relationship between PTG and FCR could be reciprocal. Research has demonstrated that positive psychology can impact cognitive evaluation procedures by offering a more positive perspective on life [42, 43]. This suggests that elevated levels of positive psychology can likewise impact patients’ perceptions of cancer and reduce the fear of the disease returning.
As FCR is negatively associated with PTG in patients with oral cancer, strategies to reduce FCR are warranted to improve PTG. Medical professionals can employ a variety of psychological therapies, including mindfulness-based stress reduction therapy, cognitive-behavioral therapy, and supportive expression therapy, to help patients reduce FCR and enhance their confidence in beating cancer.
The relationship between post-traumatic growth and coping strategies
The results of our study show a significant positive correlation between PTG and positive coping strategies and a significant negative correlation with negative coping strategies. This means that our research hypothesis H2 (Positive coping strategies are positively correlated with PTG; negative coping strategies are negatively associated with PTG) has been accepted. Patients who are able to respond to the sickness in the proper way and seek social assistance can encourage and increase their growth and mental health. Our findings support Nik’s study on patients with head and neck cancer about the relationship between PTG and coping strategies [44]. According to Choi’s cross-sectional study on Korean female cancer patients [45], positive coping is a positive predictor of PTG. Another study discovered that confrontation, which is typically regarded as a positive and helpful coping strategy, had a positive influence on PTG [46]. Research suggests that dyadic coping is a positive coping style that benefits PTG [47, 48]. Moreover, PTG benefits from positive cognitive coping strategies as well [49]. All of this research concurs that positive coping strategies have an impact on PTG ability since they might lead to the reconstruction of one’s assumptions about oneself, relationships, and spirituality and symbolize an attempt to lessen suffering. In contrast, if patients are unable to appropriately self-regulate their psychological state in order to deal with physical discomfort and psychological pressure, they may resort to negative coping such as avoidance, denial, or escapism in response to difficulties. Individuals who rely on negative coping styles often experience insufficient social support [50], which increases stress and psychological difficulties, ultimately impairing hindering positive psychological development [51]. Additionally, negative coping can promote negative psychological emotions like anxiety, fear, and unpleasantness, which are detrimental to PTG development [52, 53].
Our research findings can be explained by the stress-coping theory, which maintains that adopting positive coping strategies can lead to positive outcomes compared to negative coping strategies and that a positive assessment of stress is essential for effective stress resolution [29]. In addition, an additional explanation for this relationship could be that when an event is classified as traumatic, the affective-cognitive processing mechanism is triggered. This causes individuals to engage in rumination and contemplation of the event, which alters their negative emotional states and, in turn, their coping strategies. This coping strategy can be either good or negative, and positive coping strategies can encourage the creation of PTG, which is the core concept of Joseph’s development of the affective-cognitive processing model theory [54]. Coping strategies are also a significant predictor of PTG in Tedeschi and Calhoun’s PTG model since they are crucial for fostering personal mental health.
It is evident that positive coping strategies can foster resilience and enhance capacity to overcome adversity. Therefore, healthcare professionals should help patients develop proactive coping strategies such as confronting challenges, seeking social support, emotional regulation, and problem-solving through focused health education in order to enhance patients’ psychological resilience.
Clinical implications
First and foremost, the finding that FCR and PTG have a negative correlation opens up new possibilities for healthcare providers. For example, they can now ask patients about their thoughts on cancer recurrence, help them understand the disease, and create interventions to allay their fears and concerns. Secondly, in order to encourage healthy coping behaviors in patients with oral cancer, healthcare providers must be informed of the coping strategies used by these patients. Third, our studies have shown that the more complex the treatment, the less likely patients are to experience PTG. Therefore, healthcare professionals need to offer them greater expert assistance.
Study limitations
This design is cross-sectional. Consequently, a causal relationship between the variables and their temporal order of occurrence cannot be established. It is not possible to argue that the results related to PTG are a direct result of FCR and coping strategies. Additional longitudinal research is advised to corroborate our findings. Chinese patients from three hospitals with few sample sources participated in our study. Thus, regional variations can limit how far the research’s conclusions can be applied. In addition, when interpreting the results, it should be taken into account that the data are based on patients’ self-reports. Future studies should thus consider reports from primary caregivers. The tiny sample size of this study is one of its limitations. In order to strengthen the study’s reliability and validity of the findings, it should be considered in future research to expand the sample size.
Conclusion
To put it succinctly, our study shows that paying attention to the post-traumatic growth of patients with oral cancer is critical to enhancing their quality of life. This study demonstrates the significant influence that coping strategies and the fear of cancer recurrence have on the development of post-traumatic growth in patients with oral cancer. To stimulate post-traumatic growth, it is necessary to reduce the fear of recurrence and improve the ability to adopt proactive actions in the face of traumatic occurrences. Our study’s conclusions can help oncology nurses identify cancer patients who have greater degrees of post-traumatic growth, and they might be crucial in developing post-traumatic growth strategies for the Chinese cancer patients in the future.
Author contributions
Xin Zhang, Meng Yuan, Yufeng Yue conducted data collection. Data analysis was performed by Xin Zhang. The first draft of the manuscript was written by Xin Zhang. Xiaoyan Duan revised the manuscript. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The data can be requested from Xiaoyan Duan (zgcq686988@163.com) as an electronic file.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our study obtained informed consent from all study participants and ethical approval from the ethics committee of the Stomatological Hospital of Chongqing Medical University (no. 2023/154).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA: A Cancer. J Clin. 2017;67(1):51–64. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3.Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Absolom K. British psychosocial oncology society. Psycho-Oncology 30 years celebrations. Psychooncology. 2022;31(4):558. [DOI] [PubMed] [Google Scholar]
- 5.Sumalla EC, Ochoa C, Blanco I. Posttraumatic growth in cancer: reality or illusion? Clin Psychol Rev. 2009;29(1):24–33. [DOI] [PubMed] [Google Scholar]
- 6.Ochoa Arnedo C, Sánchez N, Sumalla EC, Casellas-Grau A. Stress and growth in cancer: mechanisms and psychotherapeutic interventions to facilitate a constructive balance. Front Psychol. 2019;10:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llewellyn CD, Horney DJ, McGurk M, Weinman J, Herold J, Altman K, et al. Assessing the psychological predictors of benefit finding in patients with head and neck cancer. Psycho-oncology. 2013;22(1):97–105. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Thong MSY, Doege D, Koch-Gallenkamp L, Bertram H, Eberle A, et al. Prevalence of benefit finding and posttraumatic growth in long-term cancer survivors: results from a multi-regional population-based survey in Germany. Br J Cancer. 2021;125(6):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tedeschi RG, Calhoun LG. Posttraumatic growth: conceptual foundations and empirical evidence. Psychol Inq. 2004;15(1):1–18. [Google Scholar]
- 10.Casellas-Grau A, Ochoa C, Ruini C. Psychological and clinical correlates of posttraumatic growth in cancer: A systematic and critical review. Psychooncology. 2017;26(12):2007–18. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Thong MSY, Doege D, Koch-Gallenkamp L, Weisser L, Bertram H, et al. Benefit finding, posttraumatic growth and health-related quality of life in long-term cancer survivors: a prospective population-based study. Acta Oncol. 2023;62(9):1124–31. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Doege D, Thong MSY, Arndt V. The relationship between posttraumatic growth and health-related quality of life in adult cancer survivors: A systematic review. J Affect Disord. 2020;276:159–68. [DOI] [PubMed] [Google Scholar]
- 13.Menger F, Mohammed Halim NA, Rimmer B, Sharp L. Post-traumatic growth after cancer: a scoping review of qualitative research. Support Care Cancer. 2021;29(11):7013–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shand LK, Cowlishaw S, Brooker JE, Burney S, Ricciardelli LA. Correlates of post-traumatic stress symptoms and growth in cancer patients: a systematic review and meta-analysis. Psychooncology. 2015;24(6):624–34. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh-Wu SF, Anglade D, Gattamorta K, Downs CA. Relationships between colorectal Cancer survivors’ positive psychology, symptoms, and quality of life. Clin Nurs Res. 2023;32(1):171–84. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Lu Y, Qin Y, Xue J, Chen Y. Post-traumatic growth and related factors among 1221 Chinese cancer survivors. Psychooncology. 2020;29(2):413–22. [DOI] [PubMed] [Google Scholar]
- 17.Hoene G, Gruber RM, Leonhard JJ, Wiechens B, Schminke B, Kauffmann P, et al. Combined quality of life and posttraumatic growth evaluation during follow-up care of patients suffering from oral squamous cell carcinoma. Mol Clin Oncol. 2021;15(3):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moye J, Jahn A, Norris-Bell R, Herman LI, Gosian J, Naik AD. Making meaning of cancer: A qualitative analysis of oral-digestive cancer survivors’ reflections. J Health Psychol. 2020;25(9):1222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nik Jaafar NR, Hamdan NA, Abd Hamid N, Rajandram RK, Mahadevan R, Zakaria H, et al. Posttraumatic growth and its association with unmet supportive care needs and fear of cancer progression among head and neck cancer patients. PLoS ONE. 2022;17(3):e0265502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebel S, Ozakinci G, Humphris G, Mutsaers B, Thewes B, Prins J, et al. From normal response to clinical problem: definition and clinical features of fear of cancer recurrence. Support Care Cancer. 2016;24(8):3265–8. [DOI] [PubMed] [Google Scholar]
- 21.Simonelli LE, Siegel SD, Duffy NM. Fear of cancer recurrence: a theoretical review and its relevance for clinical presentation and management. Psychooncology. 2017;26(10):1444–54. [DOI] [PubMed] [Google Scholar]
- 22.Ozakinci G, Swash B, Humphris G, Rogers SN, Hulbert-Williams NJ. Fear of cancer recurrence in oral and oropharyngeal cancer patients: an investigation of the clinical encounter. Eur J Cancer Care. 2018;27(1):e12785. [DOI] [PubMed] [Google Scholar]
- 23.Manne SL, Hudson SV, Preacher KJ, Imanguli M, Pesanelli M, Frederick S et al. Prevalence and correlates of fear of recurrence among oral and oropharyngeal cancer survivors. J Cancer Surviv. 2025;19(1):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eissenstat SJ, Kim S, Kim B. A Meta-Study of posttraumatic growth and coping strategies. Psychol Rep. 2024;127(4):1588–612. [DOI] [PubMed] [Google Scholar]
- 25.Knauer K, Bach A, Schäffeler N, Stengel A, Graf J. Personality traits and coping strategies relevant to posttraumatic growth in patients with Cancer and survivors: A systematic literature review. Curr Oncol. 2022;29(12):9593–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. J Pers Soc Psychol. 1986;50(3):571–9. [DOI] [PubMed] [Google Scholar]
- 27.Montero-Marin J, Prado-Abril J, Demarzo MMP, Gascon S, García-Campayo J. Coping with stress and types of burnout: explanatory power of different coping strategies. PLoS ONE. 2014;9(2):e89090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nipp RD, El-Jawahri A, Fishbein JN, Eusebio J, Stagl JM, Gallagher ER, et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer. 2016;122(13):2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folkman S. Personal control and stress and coping processes: a theoretical analysis. J Pers Soc Psychol. 1984;46(4):839–52. [DOI] [PubMed] [Google Scholar]
- 30.Tedeschi RG, Calhoun LG. The posttraumatic growth inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9(3):455–71. [DOI] [PubMed] [Google Scholar]
- 31.Xie Yaning. A preliminary study on the reliability and validity of the simplifed coping style questionnaire. Chin J Clin Psychol. 1998;6(2):114–5. [Google Scholar]
- 32.Sun P, Sun Y, Jiang H, Jia R, Li Z. Gratitude and problem behaviors in adolescents: the mediating roles of positive and negative coping styles. Front Psychol. 2019;10:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Qu Y, Zhang Y, Jin S, Wang H, Wang L, et al. Characterizing the post-traumatic growth trajectory in gastric cancer survivors: a population-based longitudinal study. Support Care Cancer. 2024;32(7):483. [DOI] [PubMed] [Google Scholar]
- 34.Sharp L, Redfearn D, Timmons A, Balfe M, Patterson J. Posttraumatic growth in head and neck cancer survivors: is it possible and what are the correlates? Psycho-oncology. 2018;27(6):1517–23. [DOI] [PubMed] [Google Scholar]
- 35.Choi SH, Lee YW, Kim HS, Kim SH, Lee EH, Park EY, et al. Development and effects of a post-traumatic growth program for patients with breast cancer. Eur J Oncol Nurs. 2022;57:102100. [DOI] [PubMed] [Google Scholar]
- 36.Fingeret MC, Vidrine DJ, Reece GP, Gillenwater AM, Gritz ER. Multidimensional analysis of body image concerns among newly diagnosed patients with oral cavity cancer. Head Neck. 2010;32(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa EF, Nogueira TE, de Souza Lima NC, Mendonça EF, Leles CR. A qualitative study of the dimensions of patients’ perceptions of facial disfigurement after head and neck cancer surgery. Spec Care Dentist. 2014;34(3):114–21. [DOI] [PubMed] [Google Scholar]
- 38.Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30:S32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahabre R, Bentley G, Poikonen-Saksela P, Mazzocco K, Sousa B, Pat-Horenczyk R. Can mindfulness facilitate posttraumatic growth in breast cancer patients? The mediating role of illness perceptions and positive emotions. J Health Psychol. 2024;29(5):438–51. [DOI] [PubMed] [Google Scholar]
- 41.Ofei SD, Teye-Kwadjo E, Amankwah-Poku M, Gyasi-Gyamerah AA, Akotia CS, Osafo J et al. Determinants of post-traumatic growth and quality of life in Ghanaian Breast Cancer Survivors. Cancer Investig. 2023;41(4):379–393. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh-Wu SF, Kauffman MA, Anglade D, Shamsaldeen F, Ahn S, Downs CA. Effectiveness of different music interventions on managing symptoms in cancer survivors: A meta-analysis. Eur J Oncol Nurs. 2021;52:101968. [DOI] [PubMed] [Google Scholar]
- 43.Sheikh-Wu SF, Gerber KS, Pinto MD, Downs CA. Mechanisms and methods to understand depressive symptoms. Issues Ment Health Nurs. 2022;43(5):434–46. [DOI] [PubMed] [Google Scholar]
- 44.Nik Jaafar NR, Abd Hamid N, Hamdan NA, Rajandram RK, Mahadevan R, Mohamad Yunus MR, et al. Posttraumatic growth and coping strategies among patients with head and neck cancer: do approach coping and avoidant coping predict posttraumatic growth over time?? Front Psychol. 2021;12:716674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi S, Kim D, Cho A, An S, Kim C, Yoo I. Pathways to post-traumatic growth in Korean female cancer patients: the mediation effects of coping strategies and resilience. Eur J Psychotraumatol. 2023;14(1):2187187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou LH, Hong JF, Qin RM, Henricson M, Stenmarker M, Browall M, et al. Post-traumatic growth and its influencing factors among Chinese women diagnosed with gynecological cancer: A cross-sectional study. Eur J Oncol Nurs. 2021;51:101903. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Wang S, Tong L, Zhuang J, Xu Y, Wu Y, et al. Relationships between body image, dyadic coping and post-traumatic growth in breast cancer patients: a cross-sectional study. Front Psychol. 2024;15:1368429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi G, Shi T, Liu Y, Cai Y. Relationships between dyadic coping, intimate relationship and post-traumatic growth in patients with breast cancer: A cross-sectional study. J Adv Nurs. 2021;77(12):4733–42. [DOI] [PubMed] [Google Scholar]
- 49.Ogińska-Bulik N, Michalska P, Juczyński Z. From negative to positive effects of secondary exposure to trauma – the mediating role of cognitive coping strategies. Med Pr Work Health Saf. 2023;74(6):449–60. [DOI] [PubMed] [Google Scholar]
- 50.Akbar Z, Aisyawati MS. Coping strategy, social support, and psychological distress among university students in Jakarta, Indonesia during the COVID-19 pandemic. Front Psychol. 2021;12:694122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Chacón M, Borda-Mas M, Chacón A, Avargues-Navarro ML. Personality traits and coping strategies as psychological factors associated with Health-Related quality of life in highly sensitive persons. Int J Environ Res Public Health. 2023;20(9):5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Folkman S, Lazarus RS, Dunkel-Schetter C, DeLongis A, Gruen RJ. Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. J Personal Soc Psychol. 1986;50(5):992–1003. [DOI] [PubMed] [Google Scholar]
- 53.Oh JM, Kim Y, Kwak Y. Factors influencing posttraumatic growth in ovarian cancer survivors. Support Care Cancer. 2021;29(4):2037–45. [DOI] [PubMed] [Google Scholar]
- 54.Joseph S, Murphy D, Regel S. An Affective–Cognitive processing model of Post-Traumatic growth. Clin Psychol Psychother. 2012;19(4):316–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be requested from Xiaoyan Duan (zgcq686988@163.com) as an electronic file.


