Abstract
Background
Herbicides are a group of substances used to control undesired vegetation in both agricultural and non-agricultural settings. They are recorded as the most consumed class among other pesticides, reaching nearly two million tons worldwide. Despite their effectiveness in weed control, the extensive utilization of herbicides has raised concerns regarding adverse effects on human health. However, comprehensive reviews addressing herbicide-related human health risks remain limited. This work aims to compile scientific evidence and possible underlying mechanisms to emphasize the hazards that need to be acknowledged, as well as to explore novel strategies for minimizing the impact on human health.
Method
Scientific data on herbicide-related human health risks, including human-related data and non-human experimental research, were retrieved from databases such as PubMed, Scopus, and Google Scholar. Pre-determined eligibility criteria were applied to select the final studies.
Result
A narrative summary of evidence-based human incidence and laboratory experiments is presented to organize and highlight key findings. This indicates the life-threatening nature of herbicide exposure in humans, ranging from acute toxicity to the development of chronic diseases at any stage of life.
Conclusion
Herbicidal chemicals can harm individuals through various pathways, especially by inducing oxidative stress or directly disrupting molecular and cellular processes. Despite some conflicting findings, effective mitigation strategies are urgently needed to promote a safer society and protect human well-being.
Keywords: Herbicide, Human health, Toxicity, Pathology, Management strategies
Introduction
As the global population is forecasted to surpass 9 billion by 2050 with the subsequent high food demands, it is essential to maximize productivity to ensure food security and cater to the needs of the growing population [1]. Herbicide, a subset of pesticides depending on their pest management, are defined as any natural or chemical compounds used to eradicate or reduce unwanted weeds that interfere with crop growth by competing for nutrients, water, and light [2]. The concept of weed management began in the late 1890s and was further introduced for general agricultural purposes in the early 1940s. The first two discovered herbicides were 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) [3]. Currently, herbicide use is not only limited to agricultural but also extend to non-agricultural applications, including aquatic weed management, wildland ecosystem, and urban areas maintenance [4–6]. Globally, herbicide exhibited the highest consumption among other pesticides due to their various advantages, such as cost-effectiveness, time and human labor savings, and high weed control efficacy [7].
However, the widespread use of these substances has raised concerns regarding their potential adverse effects on human health. Certain residues are non-biodegradable ability and persist in natural resources such as water surfaces, soil particles, and the atmosphere [8]. Herbicide-associated health problems can manifest as either acute or chronic toxicity, depending on the amount and duration of exposure. These adverse effects can occur at any age or gender. Hence, occupational, accidental, or purposeful exposures to these chemicals, whether by skin contact, ingestion, or inhalation via contaminated commodities, water, or airborne pollutants, poses a risk. Several studies have also demonstrated health implications after contact with herbicide residues, including cytotoxicity, neurotoxicity, infertility, metabolism, and endocrine disruption through various signaling pathways [9]. In addition, herbicide poisoning is related to high morbidity and mortality, which is exacerbated by the absence of specific treatments to mitigate the effects [10, 11]. Therefore, addressing human health problems related to herbicide exposure is crucial for several reasons. Understanding the potential health risks can advocate individuals to self-guard against the likelihood of residues exposure. It will also encourage workers, communities, organizations, and governments to improve environmental and social responsibility by adopting best practices and creating legislation to prevent unacceptable residue pollution. Furthermore, these insights provide a proactive basis for research and development, leading to the advancement of safer alternatives and management strategies.
Although the widespread use of herbicides and their life-threatening matter have recently been a major concern, comprehensive reviews on their potential health risks remain limited. This review aims to compile the human health problems caused by herbicide exposure. Evidence-based epidemiology studies, case reports, and laboratory experiments exploring the possible underlying mechanisms that contribute to pathological diseases are provided to emphasize the hazard that needs to be concerned. In addition, evidence-based strategies and management approaches to alleviate their adverse effects on human health are also discussed.
Methods of literature search
Database search and keyword selection
A comprehensive search for relevant articles was conducted across several academic databases, including PubMed, Scopus, and Google Scholar. To ensure a thorough capture of pertinent literature, the following keywords were employed, either alone or in any combination: “herbicide” OR “herbicides” OR “glyphosate” OR “paraquat” OR “atrazine” OR “2,4-D” OR “2,4-dichlorophenoxyacetic acid” with “consumption” OR “utilization” OR “exposure” OR “toxicity” “side effects” OR “human health” OR “health effects” OR “epidemiology” OR “toxicological impact” OR “mitigation strategies” OR “implementation”. The search was limited to articles published in English, with no restriction on the publication year.
Article selection criteria
The primary focus was on studies investigating the adverse effects of herbicide exposure on both the general population and herbicide applicators. Articles related to human incidence and herbicide exposure, including systematic reviews, meta-analyses, case reports, cohort studies, and case-control studies, were selected regardless of whether the outcomes were statistically significant. Additionally, non-human studies providing valuable insights into potential human health complications were also selected. Experiments conducted with non-human subjects were included if they demonstrated mechanisms or biomarkers that could be replicated in humans. Additionally, studies that ensured the observed toxicity was attributed to the pure chemical herbicide itself, rather than to any co-adjuvants, were considered.
Overview of herbicide application
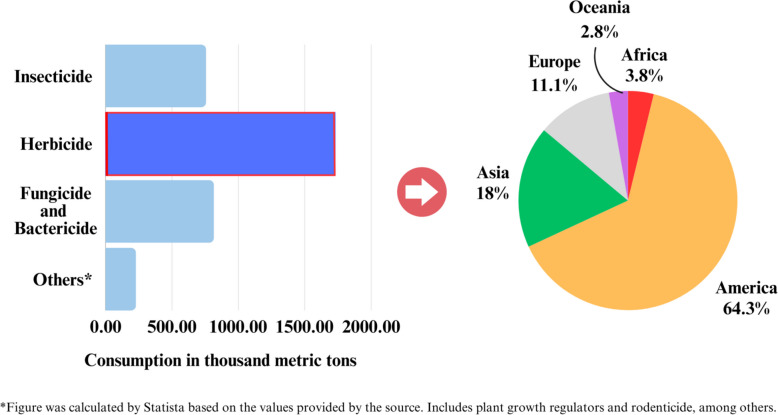
In 2021, herbicide consumption was estimated to have exceeded 1.7 million tons globally, outpacing all other types of pesticides used for agricultural purposes (Fig. 1) [7]. In addition, its applications beyond agriculture for aquatic weed management, public wildland management, and urban area maintenance [4–6]. The top three continents showing the highest herbicide consumption were the Americas, Asia, and Europe, accounting for more than 1 million, 300 thousand, and 190 thousand tons, respectively [12]. Brazil, one of the world’s prominent producers of major commodities including soybeans, coffee, and corn [13], applied approximately 400 thousand tons, which almost doubled in the past decade [12]. During this period, herbicides also remained the most used class, accounting more than 50% of total pesticides, as the global trend. The top active ingredients were glyphosate (GLY), 2,4-D, atrazine (ATZ), and paraquat (PQ), accounting for 36%, 7.4%, 4.6%, and 1.5%, respectively [14]. The United States of America was the leader of herbicide utilization at approximately 300 thousand tons in 2021 [12]. GLY was most widely used in the country, with approximately 140 thousand tons applied annually [15], followed by ATZ, 2,4-D, and PQ at nearly 43, 25, and 6 thousand tons, respectively [16–18].
Fig. 1.
Global pesticides consumption with particular herbicide usage across different continents as recorded in 2021 (Data obtained from Statista [7] ; FAOSTAT [12])
Among Asian countries, China had the highest herbicide consumption at almost 100 thousand tons, due to 10% crop yield loss and a 60 million tons reduction in grain output were caused by weed damage. There were more than thousands of product registrations in 2021, in which the highest registered active ingredients were GLY (1,805 products), ATZ (1,401 products), and acetochlor (1,238 products) [19]. Almost half of the herbicide consumption in Asia was contributed by the Southeastern region, especially Thailand, Vietnam, and Malaysia [12, 20]. Since the agricultural sector is fundamental to the national and regional economy, herbicide is the common weed control used to meet global demands. In Thailand, yearly pesticides imports were comprised of herbicide accounting for 80%. Notably, 79% of the total were GLY (40–50%), PQ (15–20%), and 2,4-D (11–18%), as recorded in 2021 [21]. On the other hand, herbicide utilization in Europe is less significant. The percentage value shared in the market was 34% herbicide, which was second only to fungicide and bactericide holding 44% [22]. As illustrated, Africa and Oceania were the two continents with the smallest herbicide consumption with 3.8% and 2.8% respectively.
Herbicide exposure and impacts on human health
The widespread utilization of herbicides allows their residues to enter environmental resources through transfer processes, including adsorption, runoff, or volatilization, resulting in bioaccumulation where specific residues remain persistent in nature [23]. Thus, exposure to residues can occur through various transmission routes, including skin contact, inhalation, ingestion, and in various situations, such as self-harm, accidental exposure, contaminated food consumption, or occupational contact (i.e., agriculture, horticulture, and industrial production). The primary route through which humans come into contact with herbicides is skin exposure, which often occurs during the preparation, application, and even cleanup of contaminated utensils and protective clothing. The ability of chemicals to penetrate the skin depends on their formulations. For instance, oil- or solvent-based liquid formulations are more easily absorbed compared to water-soluble wettable powders, dusts, and granular formulations. In addition, the area of skin contact, duration of exposure, and chemical intensity also influence the severity of dermal exposure [24]. Ocular exposure poses a significant risk, as the eyes are highly absorbent tissue that can easily allow chemicals to enter the bloodstream, causing irritation or blindness in severe conditions. Exposure can occur through airborne particles, splashes during application, or by rubbing the eyes with contaminated hands or gloves. Inhalation of vapors, powders, or aerosols particles can result from burning containers, pouring, spraying herbicide, or using methods that generate smaller droplets such as high pressure, ultra-low volume, or foggy equipment [25]. Ingestion of herbicide typically occurs accidentally or as a result of suicidal attempt. Activities such as unplugging the nozzle with mouth, smoking, eating with unwashed hands after application, as well as consuming the commodities containing residues above regulation levels allow the particles to enter the body. Moreover, children are frequently the victims of unintentionally intake of improperly stored products, and unlabeled bottles or food containers [24]. These exposures drive the off-target consequences, which negatively affect humans. The herbicides, notably GLY, PQ, 2,4-D, and ATZ, which are consistently prevalent in most countries and possess several threats to human health causing high morbidity and mortality, will be the focus of this review. This included major evidence supporting their potential adverse effects on human health both in terms of acute toxicity and the development of chronic diseases (Fig. 2).
Fig. 2.
Herbicide exposure and possible health impacts (By author)
Acute toxicity
Acute toxicity is defined as adverse effects that occur within a short time, typically observed within 14 days after exposure to a single dose or multiple doses of a substance lasting less than 24 h [26]. According to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) classification, acute toxicity is categorized into five levels, with class 1 representing the most severe hazard. These categories are based on animal testing via oral, dermal, and inhalational routes [27]. GLY, ATZ, and 2,4-D are categorized into class 4 for an acute oral toxicity with the observed lethal doses between 300 and 2,000 mg/kg [28–30]. Meanwhile, PQ is placed into class 3 due to its higher oral acute toxicity, with observed lethal doses between 50 and 300 mg/kg [31]. Moreover, most of the herbicides are classified as Category 1 for acute dermal toxicity, indicating severe skin and eye irritation [28–31]. In particular, PQ can cause severe poisoning even through damaged skin or prolonged skin exposure [32].
Indeed, the impact of acute toxicity in real-life scenarios can be represented through suicidal attempts or occupational exposure. Approximately 20% of suicidal cases are specifically attributed to pesticides self-poisoning [33]. Herbicides are the most commonly used group among individuals, with PQ being the leading chemical, followed by GLY [34]. As detailed in Table 1, the initial clinical features of herbicide poisoning are non-specific, such as excessive salivation, nausea, vomiting, drowsiness or mucosal burns. Several GLY-poisoning cases presented rapid onset of multiorgan failure following the ingestion of large quantities approximately 75–500 mL of 30–41% GLY solution [35–39]. Symptoms of GLY poisoning commonly include electrolyte abnormality, circulatory shock, gastrointestinal tract hemorrhage, respiratory failure, renal failure, cardiac arrest and ultimately death in severe cases [35–39].
Table 1.
Herbicide-associated acute toxicity
| Herbicide | Subjects | Dose Exposure | Main findings | Ref. |
|---|---|---|---|---|
| GLY | Case report | 200 mL of 30–36% GLY | Respiratory distress, hypotension, metabolic acidosis, and hyperkalemia were noted during hospitalization, but was successfully resuscitated. | Mutkule, Venkategowda, and Mahendrakar [35] |
| 400 mL of 41% GLY | Metabolic acidosis, respiratory failure and shock were noted during hospitalization, ultimately leading to a fatal outcome. | C.-B. Chang and Chang [36] | ||
| 200 mL of 36% GLY | Metabolic acidosis, cardiorespiratory deterioration, erosive lesions of digestive tract, acute renal failure, and circulatory shock were noted during hospitalization, but was successfully resuscitated. | Picetti et al. [37] | ||
| 500 mL of 41% GLY | Respiratory distress, hypotension, metabolic acidosis, and pulmonary consolidation were noted during hospitalization, but was successfully resuscitated. | Kunapareddy and Kalisetty [38] | ||
| 75 mL of 41% GLY | Epigastric tenderness, respiratory distress, metabolic acidosis, and acute pulmonary edema were noted during hospitalization, but was successfully resuscitated. | Khot et al. [39] | ||
| PQ | Case report | 10 mL of 24% PQ | Oral ulcers, hypokalemia, and renal failure were noted during hospitalization, but was successfully resuscitated. | Janeela et al. [40] |
| Unknown quantity of 24% PQ | Congested and edematous oral mucosa, tachycardia, respiratory alkalosis, erosive lesions of digestive tract, blood-mixed bronchoalveolar lavage fluid, and renal failure were noted during hospitalization, but was successfully resuscitated. | Ghosh et al. [41] | ||
| 10 mL of 20% PQ | Oral ulcers, metabolic acidosis, and acute kidney and liver injury were noted during hospitalization, but was successfully resuscitated. | Sigdel et al. [42] | ||
| > 100 mL of 20% PQ | Oral ulcers, and severe lung, liver, and kidney damage were noted during hospitalization, but was successfully resuscitated. | Yu et al. [43] | ||
| 20% w/v PQ | Erythema, blister, and severe haemorrhaging diathesis on exposed skin, with dyspnea in some cases. | Zhou et al. [44] | ||
| 2,4-D | Case report | 30 mL of 58% 2,4-D | Oral secretion, tachycardia, poor muscle response, renal failure, and circulatory collapse were noted during hospitalization, and ultimately leading to a fatal outcome. | Demissie et al. [45] |
| 70 mL of 58% 2,4-D | Tachycardia, metabolic acidosis, and diffuse cerebral edema were noted during hospitalization, but was successfully resuscitated. | Hiran and Kumar [46] |
Similarly, individuals who consumed nearly 10–100 mL of a 20–24% PQ solution presented signs of multiorgan failure encompassing renal, hepatic, respiratory, and cardiac dysfunction [40–43]. Particularly, the preceded diffuse alveolitis and subsequent pulmonary fibrosis are the hallmarks of PQ poisoning [41, 47]. In addition to suicidal attempted cases, the workers who sprayed a concentrated formulation of 20% weight per volume (%w/v) of PQ for approximately 3 h a day, without any protection, exhibited erythema, blister, and severe haemorrhaging diathesis on their skin [44]. Moreover, some cases of PQ poisoning through skin contact exhibited dyspnea as an atypical complication, accompanied by abnormal breath sounds [44]. These conditions suggest that PQ can be absorbed through the skin and spread throughout the body, causing life-threatening effects in humans. In addition, severe cases who ingested between 30 and 70 mL of 58% 2,4-D progressed into comatose state by developing circulatory collapse, poor muscle response, or ultimately death despite receiving several supportive cares [45, 46].
Unfortunately, the specific antidote for pesticides poisoning is only available for carbamate and organophosphate poisoning [48]. Therefore, in all of the herbicide-poisoning cases, the mainstay of management includes decontamination, symptomatic and supportive care. The individual prognosis then relies on dose dependence, promptness of medical intervention and effectiveness of immediate resuscitation [46]. Collectively, despite being considered of low acute toxicity in humans, exposure to concentrated solutions can result in severe toxicity or even mortality. The adverse effects may not be confined to the specific body part of exposure, but can extend to other systems throughout the body, highlighting the potential for life-threatening consequences.
Chronic toxicity
Chronic toxicity refers to the sublethal effects, as well as lethal effects in some cases, that a substance can have on living organisms when they are repeatedly exposed over a period of time, usually at lower or less toxic concentrations compared to acute toxicity tests [49]. Major herbicide-related sublethal effects include neurodegenerative disorders, cancer, reproductive system disorders, congenital disorders, respiratory system disorders, and immunological disorders. This section describes the principal effects contributing to pathological diseases and highlights the primary toxic herbicide affecting each body system, as established by human incidence and experimental studies.
Neurodegenerative disorders
Although the etiology of neurodegenerative disorders such as Parkinson’s (PD), Alzheimer’s (AD), and Huntington’s disease (HD) remains multifaceted, neurotoxic agents like herbicide, which disrupt the function or alter the chemical levels of the nervous system, are significant contributors, as evidenced in Table 2 [50, 51]. Specifically, a study reported that occupational uses of pesticides increased the risk of PD by 29 to 89%, doubling for those who used them for more than 10 years. Among the 360 participants diagnosed with PD, 11.4% were associated with herbicide exposure, including 2,4-D, GLY, and PQ [52]. While most studies on human incidences found a positive correlation between AD development and pesticide exposure, they were also acknowledged for having small number of cases. For instance, one study reported that 344 out of 3,084 participants developed AD following pesticide exposure [53]. Moreover, the explorations did not thoroughly investigate specific types of pesticides, leading to the ambiguous toxicity of herbicide-induced AD [54, 55]. Furthermore, there is a lack of epidemiological evidence connecting between herbicide exposure to HD development, necessitating future research in this area.
Table 2.
Herbicide-associated neurodegenerative disorders
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| PQ | C57BL/6J mice | 10 mg/kg |
- Reduced motor control through dopaminergic neuron loss, lipid profile alteration, inflammation activation, and PD-related gene malfunction. - Induced DNA 8-oxodG in the brain, heart, and small intestine. |
De Luca et al. [56] and Tong et al. [57] |
| Human osteosarcoma cells | 30 µM | Induced the aggregation of Huntington protein and cell death through disruption of protein degradation pathways. | Pinho et al. [58] | |
| GLY | C57BL/6J mice | 125 to 500 mg/kg/day | Induced neuroinflammation through the upregulation of TNF-α cytokine. | Winstone et al. [59] |
| APP/PS1 pups primary cortical neurons | 10 to 40 µg/mL | Induced the expression of soluble Aβ40−42 and decreased cell viability. | ||
| Induced pluripotent stem cells (iPSCs) | 0.1 to 1000 µM | Altered metabolic activity and enhanced permeability of the blood-brain barrier through the disruption of occludin and claudin-5 proteins. | Martinez and Al-Ahmad [60] | |
| Triazine group | In silico study | - | Disrupted cell cycling and cellular senescence processes through targeting EGFR, FN1, and TYMS proteins. | Li et al. [61] |
However, PQ is a well-known neurotoxin, particularly associated with PD development, due to its structural similarity to dopaminergic neurotoxin like 1-methyl-4-phenylpyridinium (MPP+). In addition to oxidative stress induction and mitochondrial damage [62], PQ-treated mice showed the disruption of lipid metabolism in the midbrain, leading to motor dysfunction and increased levels of inflammatory cytokines (i.e., interleukins (IL-6 and IL-1β), which significantly correlated with PQ-poisoning cases in humans [57]. Furthermore, this herbicide has also been implicated in HD development. De Luca et al. [56] demonstrated that PQ-treated mice exhibited the formation of oxidized purine 8-oxo-7,8-dihydroguanine (8-oxodG), a biomarker of DNA oxidation commonly found in post-mortem brains of HD patients. Additionally, Pinho et al. [58] found that PQ-conjugated mitochondria increased mutant huntingtin (mHTT) aggregation and cell death in human osteosarcoma cells. These findings suggest that mitochondria-derived reactive oxygen species (ROS) may impair protein degradation pathways, contributing to mHTT aggregation and HD development.
Conversely, GLY and triazine herbicide have been possibly linked to AD development. Studies have demonstrated that GLY can penetrate the blood-brain barrier, by impairing tight junction proteins like occluding and claudin-5, and inducing inflammatory responses in both mice and human neurons [59, 60]. This inflammation is also associated with alterations in AD-related pathological markers, such as β-amyloid (Aβ) levels and cell viability. Together, Li et al. [61] identified triazine herbicide, including ATZ, simazine (SIM), and propazine (PRO), as potential contributors to AD through in silico analysis. Disruption of cell cycle regulation and cellular senescence by targeting thymidylate synthase (TYMS), fibronectin 1 (FN1), and epidermal growth factor receptor (EGFR), leading to abnormal development and cell death of neuronal cells, were proposed as underlying mechanisms. Although these findings are preliminary, they lend support to the hypothesis that herbicide exposure could contribute to AD development.
Cancer
Extensive research has been conducted for decades on the correlation between pesticide exposure in agriculture, horticulture, forestry, pesticide manufacturing, and cancer development [63]. Table 3 provides various types of cancer that have been linked to herbicide exposure, including gastric (odds ratio (OR) = 1.66, 95% confidence interval (CI): 1.08–2.55), thyroid (OR = 3.00, 95% CI: 1.38–6.54), bladder (rate ratios (RR) = 1.54, 95% CI: 1.05–2.26), lung (RR = 1.74, 95% CI: 1.07–2.84), colorectal (RR = 1.75, 95% CI: 1.08–2.83), endometrial (OR = 5.25, 95% CI: 1.84–17.67), and brain tumors (effect sizes (ES) = 2.38, 95% CI: 1.31–4.33) [64–69].
Table 3.
Herbicide-associated cancer
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| Not specifically identified | Human study | - | Significantly associated with 1.5 to 2.0-fold higher risk of diffuse-type gastric cancer. | Shah et al. [64] |
| Significantly associated with an increased risk of thyroid cancer. | Han, Kim, and Song [65] | |||
| Significantly associated with endometrial cancer in agricultural, poultry, livestock exposure. | Peñalver-Piñol et al. [67] | |||
| Significantly associated with brain tumor in childhood domestic exposure. | Onyije et al. [69] | |||
| Acetochlor | Human study | - | Significantly increased risk of colorectal cancer in high lifetime users and lung cancer in ever and low lifetime users. | Lerro et al. [68] |
| Imazaquin and Imazethapyr | Human study | - | Significantly increased risk of bladder cancer, especially among non-smokers. | Koutros et al. [66] |
| GLY | Human study | - | Significantly associated with NHL incidence. | De Roos et al. [70] |
| Significantly increased risk of follicular lymphoma. | Meloni et al. [71] | |||
| Significantly associated with detectable mosaic loss of chromosome Y in high lifetime users. | Chang et al. [72] | |||
| 2,4-D | hDPSCs | 0.1 µM to 10 mM | Induced oxidative stress and apoptosis in subjects. | Mahmoudinia et al. [73] |
| Male Wistar rats | 20 to 52 ppm | Induced hyperkeratosis in esophageal epithelium cells, and mild dysplasia in small and large intestine. | Mariotti et al. [74] | |
| 15, 75 and 150 mg/kg/day | Reduced the body weight, increased liver weight, and hypertrophy of heart, and induced oxidative stress and lipid peroxidation in erythrocytes. | Wafa et al. [75] | ||
| ATZ | Human study | - | Significantly associated with NHL incidence. | Remigio et al. [76] |
| PQ | Human study | - | Significantly increased the risk of NHL in users. | Park et al. [77] |
| Male Wistar rats | 30 to 200 mg/kg/day | Induced pathological changes and cell death in lung, kidney, and liver tissue, as well as DNA damage in peripheral lymphocytes through oxidative stress. | Alizadeh et al. [78] | |
| HeLa and Hep G2 cell lines and human peripheral lymphocytes | 50 to 300 µM | Induced strand break and oxidized bases of DNA under certain concentration and time of exposure depend on cell types. | Petrovská and Dušinská [79] |
Despite ongoing debates regarding the carcinogenicity of GLY [80, 81], the International Agency for Research on Cancer (IARC) has classified it as probably carcinogenic to humans (Group 2 A) [82]. Study has linked GLY exposure to a significantly elevated risk of non-Hodgkin lymphoma (NHL) (OR = 2.02, 95% CI: 1.10–3.71) [70]. Also, a multicenter case-control study conducted by Meloni et al. [71] found a significant association between GLY exposure and a 7-fold increased risk of follicular lymphoma. In addition to its previously documented role in oxidative stress-induced cancer [83], Chang et al. [72] identified mosaic loss of chromosome Y (mLOY) as another potential mechanism contributing to hematologic cancers and solid tumors in GLY-intoxicated male applicators. Among participants, 21.4% had detectable mLOY, and 9.8% had expanded mLOY greater than 10% of cells. These findings suggest that GLY may induce genomic instability and genotoxicity, resulting in certain hematological cancers.
Meanwhile, 2,4-D has been classified as possibly carcinogenic to humans (Group 2B) due to its inadequate evidence in humans [84]. Epidemiological studies among 1,000 2,4-D factory workers have also not found a significant increase in overall cancer incidence [85]. However, its carcinogenicity should not be neglected. Several studies have consistently linked 2,4-D exposure to oxidative stress, a known cancer-driving factor [73, 75, 86, 87]. This effect is often mediated through the generation of free radicals from lipid peroxidation and/or the attenuation of antioxidant properties [75]. Malondialdehyde (MDA), a marker of lipid peroxidation, has been found to be elevated in 2,4-D-treated mice [75, 86, 87] and human dental pulp stem cells (hDPSCs) [73]. Moreover, Mariotti et al. [74] observed gastrointestinal changes in 2,4-D-treated rats, including esophageal hyperkeratosis and mild dysplasia in both small and large intestines, suggesting that intestine may be particularly susceptible to the carcinogenic effects of 2,4-D.
Among commonly used herbicides, ATZ has been classified as "not classifiable as to its carcinogenicity to humans" (Group 3) [88]. Despite being widely recognized as an endocrine-disrupting chemical, its ability to induce hormonally sensitive cancer in animals has not been replicated in humans. Studies have reported the absence of intrinsic estrogenic activity in ATZ, leading to uncertainty regarding its carcinogenic potential. However, ATZ exposure was linked to an increased risk of certain non-hormonally sensitive cancers such as lung cancer (RR = 1.24, 95% CI: 1.04–1.46) and NHL (RR = 2.43, 95% CI: 1.10–5.38) in a study examining over 50,000 pesticides applicators [76]. Meanwhile, PQ has not yet been evaluated by IARC for its carcinogenicity. Nevertheless, a study of over 20,000 participants suggested a potential increased risk of NHL among those exposed to PQ (RR = 1.51, 95% CI: 1.01–2.26) [77]. In addition, there is little firm evidence demonstrating its potential to induce oxidative stress related-DNA damage or genotoxicity. Studies conducted by Alizadeh et al. [78] and Petrovská and Dušinská [79] showed that PQ-induced oxidative stress caused DNA strand breakage, oxidation of DNA bases, and impairment of cell membrane integrity, observed in peripheral lymphocytes of male Wistar rats, isolated human peripheral lymphocytes, and human cell lines. Additionally, molecular docking studies further revealed that the interaction between PQ and H4 histone proteins may disrupt the arrangement of nucleosomes, affecting DNA packaging, chromosomal formation, and genomic stability, potentially leading to carcinogenic effects [89].
Reproductive system disorders
Endocrine disruption typically leads to reproductive system disorders, as hormones are essential for the growth and function of this system [90]. Thus, exposure to endocrine-disrupting herbicides has raised concerns regarding potential adverse effects, as evidenced in Table 4.
Table 4.
Herbicide-associated reproductive system disorders
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| ATZ | Human study | - | Significantly associated with menstrual cycle length irregularity, reduced reproductive hormone levels and longer follicular phase. | Cragin et al. [91] |
| Male and female crayfish | 5 to 20 mg/L | Altered both testis and ovary cellular structure, reduced testosterone level, and enhanced estradiol and progesterone levels. | Nassar et al. [92] | |
| Rat granulosa cells | 10 and 20 µM | Inhibited FSH-induced LHR, CYP19A1 mRNA expression and estradiol production through the activation of ERK1/2 signaling pathway. | Fa et al. [93] | |
| Sprague-Dawley and Long Evans female rats | 50 to 100 mg/kg | Inhibited LH surge and reduced the number of corpus luteum and ova shed. | Foradori et al. [94] | |
| Wistar male rats | 120 mg/kg | Induced testicular defects through oxidative stress induction and endocrine disruption. | Rotimi, Ojo, and Adeyemi [95] | |
| Rat granulosa cells, and H295R adrenal cortical carcinoma cells | 1 to 30 µM | Increased estradiol, estrone, and progesterone production, and aromatase activity. | Tinfo et al. [96] | |
| Human granulosa-luein cells, and endometrial stromal cells | 1 nM to 0.1 mM | Increased aromatase activity in granulosa-lutein cells. | Holloway et al. [97] | |
| In silico study | - | Significantly enriched of miscarriage genes. | Harris et al. [98] | |
| 2,4-D | Kunming male mice | 50 to 200 mg/kg/day | Disrupted spermatogenesis, and induced testicular damage through endocrine disruption, oxidative stress and apoptosis induction. | Zhang et al. [86] |
| Wistar male rats | 100 and 200 mg/kg | Altered testicular, seminal vesicles and prostate weight and histology though hormonal (testosterone, FSH, and LH) alteration. | Marouani et al. [99] | |
| Nulliparous Wistar female rats | 70 mg/kg/day | Increased ROS level, lipid peroxidation, and protein oxidation in ventral prostate, ovary, and breast but different pattern depending on ages of subjects. | Pochettino et al. [87] | |
| PQ | Sprague-Dawley male rats | 0.5 to 8 mg/kg | Detained Leydig cell regeneration through ROS induction and growth factor, and DHH down-regulation. | Li et al. [100] |
| CD-1 female mice | 120 mg/mL | Impaired oocyte quality through the disruption of nuclear and cytoplasmic maturation during oocyte meiosis. | Sun et al. [101] |
ATZ is a notable herbicide that primarily harms the reproductive system and developing organisms through endocrine disruption [102]. A study reported that women exposed to ATZ in municipal drinking water was associated with menstrual cycle length irregularity (OR = 6.16, 95% CI: 1.29–29.38) [91]. It targets the hypothalamus-pituitary-gonadal (HPG) axis such as the inhibition of the brain-pituitary-ovarian alliance [91–93, 95, 103]. In vivo studies also demonstrated that ATZ caused histological changes and affected the functions of the testis, ovary, and epididymis, confirmed by significant declines in sperm quality, the number of ovulated follicles, hormone production, including testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), and the preovulatory LH surge [94, 95]. Similarly, ATZ-treated granulosa cells showed an increased aromatase activity, an enzyme that converts androgens into estrogens, suggesting a potential for hormonal imbalance [96, 97]. Moreover, it was the only herbicide identified as being associated with an increased risk of miscarriage in multiple species, including human, mouse, and rat [98]. Although Goodman et al. [104] conducted a systematic review across 21 epidemiological studies to support the association between ATZ exposure and adverse pregnancy outcomes, the low quality of the available data, mostly from aggregate rather than individual information, made it difficult to establish a definitive causal relationship. Therefore, the clinical evidence remains limited, pointing out the need for future research.
Meanwhile, 2,4-D and PQ have similar effects on the reproductive system, mainly through the induction of oxidative stress and apoptosis. 2,4-D-induced oxidative stress in mice exhibited signs of testicular damage, including atrophy of the seminiferous tubules, disorganization of seminiferous epithelium, enlarged intracellular space, and loss of gamete cells [86, 99]. Although hormone disruption was not observed in 14 consecutive days, a decrease in testosterone levels and an increase in FSH and LH serum levels were evident after 30 days of exposure [86]. Pochettino et al. [87] found that 2,4-D induced oxidative stress in prostate tissue at all ages. However, breast tissue, especially during puberty and adulthood, was more susceptible to oxidative imbalance, while the oxidative status in the ovary exhibited age-dependent effects. Regarding PQ toxicity, Li et al. [100] demonstrated that PQ delayed Leydig cell development and reduced testosterone production by disrupting Sertoli cell function through the downregulation of SRY-box transcription factor 9 (SOX9) and desert hedgehog (DHH). PQ also impaired oocyte maturation by interfering with spindle assembly and kinetochore-microtubule attachment during meiosis, causing misaligned chromosomes and triggering mitochondrial distribution [101]. In addition, as reviewed by Milesi et al. [105], GLY exposure has been linked to detrimental effects on reproductive health as well as on pre- and post-implantation embryos, through the alterations in estrogen biosynthesis, estrogen receptor expression, and signaling pathways crucial for the implantation process.
Congenital disorders
Genetic abnormalities noteworthily contribute to pathological processes of congenital disorders, but maternal exposure to agrichemicals during pregnancy is one of the driving factors [106]. Herbicide and its potential on congenital disorders are provided in Table 5. Although the Food and Agriculture Organization (FAO) has concluded that ATZ was not a teratogen [107], it has been linked to birth defects (i.e., gastroschisis and choanal atresia) in infants born to mothers residing near high ATZ-contaminated areas [108, 109]. A study by Lee et al. [110] also linked low thyroxine levels to an increased risk of choanal atresia in newborns (adjusted odds ratio (AOR) = 0.85, 95% CI: 0.80–0.90). This finding aligns with the hypothesis that ATZ exposure may disrupt thyroid hormone function. In ovo studies demonstrated that ATZ can cause thyroid gland disorganization, characterized by an enlarged thyroid stromal compartment, increased interstitial collagen content, and follicular hyperplasia as well as decreased expression of androgen receptors in the follicular epithelium [111].
Table 5.
Herbicide-associated congenital disorders
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| ATZ | Human study | - | Significantly associated between maternal exposure and gastroschisis in offspring, especially those who resided less than 25 km from contaminated area. | Waller et al. [108] |
| Human study | - | Significantly associated between maternal exposure and the risk for choanal atresia and stenosis in offspring. | Agopian et al. [109] | |
| C. latirostris | 0.2 ppm | Altered thyroid histo-function in embryo, and affected the growth curve from hatching. | Galoppo et al. [111] | |
| 2,4-D | Human study | - | Significantly associated between maternal exposure and slower auditory signal transmission in newborn. | Silver et al. [112] |
| Bullfrog embryos and tadpoles | 150 to 1200 mg/L | Inhibited embryonic growth and induced physical deformation. | Viriato et al. [113] | |
| Rhinella arenarum embyos | 1 to 15 mg/L | Reduced body size, delayed development, microcephaly, agenesis of gills, and abnormal cellular proliferation processes of embryos. | Aronzon et al. [114] | |
| GLY&PQ | Human study | - | Pregnant women who work or are involved in agricultural activities exhibited detectable residue levels in maternal serum and umbilical cord blood. | Kongtip et al. [115] |
| GLY | ddY mice | 0.098% w/v | Maternal exposure led to autism-like behavior and abnormal composition of gut microbiota in offspring through increased of soluble epoxide hydrolase (sHE) activity. | Pu et al. [116] |
A Chinese cohort study by Silver et al. [112] found that prenatal 2,4-D exposure linked to slower auditory signal transmission in newborns, potentially due to impaired myelination of the auditory pathway. Other teratogenic effects of 2,4-D were also observed in vivo [113, 114]. For instance, 2,4-D-treated bullfrog embryos exhibited deformities and developmental delays [113]. Furthermore, Kongtip et al. [115] detected both GLY and PQ in the serum of Thai female agricultural workers during delivery. Although this study did not assess the impact on infants, the presence of these chemicals raises concern about potential exposure through lactation, given findings by Pu et al. [116] demonstrating that maternal exposure to GLY during pregnancy and lactation caused autism spectrum disorder (ASD) in offspring.
Diabetes mellitus (DM)
Surprisingly, a recent case-control study among 2,626 participants Chinese population by Wei et al. [117] exhibited a positive association between plasma herbicide and the risk of DM type 2. The study by Saldana et al. [118] also found that 4.5% of 11,273 pregnant women had the risk of gestational DM after exposure to ATZ, butylate, 2,4,5-T, and 2,4,5-(trichlorophenoxy)propionic acid (2,4,5-TP) during the first trimester of pregnancy (OR = 2.2, 95% CI: 1.5–3.3). Starling et al. [119] additionally reported an association between farmer’s wives who exposed to 2,4,5-T and 2,4,5-TP and the development of DM during a 10-year follow-up period (Hazard ratios (HR) = 1.59, 95% CI: 1.00–2.51). These observations have encouraged other studies to establish a causal relationship between herbicide exposure and the likelihood of DM development, as shown in Table 6.
Table 6.
Herbicide-associated diabetes mellitus
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| Not specifically identified | Human study | - | Significantly associated between plasma herbicide and fasting plasma glucose, fasting insulin and HOMA2-IR (insulin resistance), indicating an increased risk of T2DM. | Wei et al. [117] |
| ATZ, butylate, 2,4,5-T, and 2,4,5-TP | Human study | - | Significantly associated with the risk of gestational DM in pregnant women who exposed to these herbicides in agricultural activities. | Saldana et al. [118] |
| 2,4,5-T, and 2,4,5-TP | Human study | - | Significantly associated with incident DM during a 10-year follow-up period in farmers’ wives who exposed to these herbicides in agricultural activities. | Starling et al. [119] |
| GLY | Wistar albino male rats | 50 to 250 mg/kg | Increased fasting plasma glucose and insulin levels by inducing oxidative stress and disrupting glucose homeostasis in the liver through NFκB signaling pathway. | Prasad et al. [120] |
| Wistar albino male rats, and in silico study | 50 to 250 mg/kg | Increased fasting plasma glucose and insulin levels through IRS-1/PI3K/Akt signaling pathway resulting in reduced GLUT4 uptake and insulin resistance. | Jayaraman et al. [121] | |
| ATZ | D. melanogaster | 2 and 20 µg/mL | Increased hemolymph glucose and trehalose levels and induced insulin resistance through oxidative stress mediated JNK signaling pathway. | Gupta et al. [122] |
| Sprague-Dawley male rats | 30 and 300 µg/kg | Increased fasting plasma glucose and induced insulin resistance through the disruption of mitochondrial function resulting in Akt-signaling pathway inhibition. | Lim et al. [123] | |
| PQ | Rat hepatocytes | 2 and 5 mM | Impaired insulin-dependent mTOR activation by reducing IGFBP-1 gene expression through the induction of oxidative stress. | Kimura et al. [124] |
| 3T3-L1 adipocytes | 5 or 10 mM | Reduced glucose uptake by repressing the basal PI3-kinase activity through oxidative stress induction. | Shibata et al. [125] |
GLY has been shown to activate the nuclear factor kappa B (NFκB) signaling pathway, leading to inflammation and insulin resistance in the liver [120]. Moreover, it induced abnormal glucose uptake in skeletal muscles by disrupting the insulin signaling pathway through protein kinase B (Akt) and insulin receptor substrate (IRS), ultimately reducing glucose transporter type 4 (GLUT4) translocation [121]. Meanwhile, both Gupta et al. [122] and Lim et al. [123] found that ATZ induced mitochondrial dysfunction and insulin resistance through activation of the c-Jun N-terminal kinase (JNK) signaling pathway or suppressing insulin-mediated Akt phosphorylation. On the other hand, PQ-induced oxidative stress inhibited the repression of insulin-like growth factor-binding protein-1 (IGFBP-1) gene expression, leading to increased hepatic glucose production and insulin resistance [124]. In 3T3-L1 adipocytes, PQ-induced oxidative stress also disrupted the binding of phosphatidylinositol 3-kinase (PI3K) to IRS, inactivating the downstream signaling pathway and reducing GLUT4 translocation and glucose uptake [125].
Interestingly, different outcomes of the 2,4-D-altered glucose metabolism were observed. Despite the unstated underlying mechanism, preliminary findings in mice showed a decrease in blood glucose levels without changes in liver function or amylase activity [126]. Furthermore, Sun et al. [127] proposed a novel mechanism involving the induction of the peroxisome proliferator-activated receptor (PPARβ), which was upregulated in a dose-dependent manner, ultimately enhancing glycogen accumulation.
Respiratory system disorders
As shown in Table 7, several investigations revealed a significant relationship between herbicide exposure and its effects on respiratory health. The cross-sectional study among 180 Thai herbicide applicators found a vital prevalence of wheeze and dyspnea, especially in those spraying more than 150 L of chemicals a day, using multiple types of herbicides and improper handling without wearing personal protective equipment (PPE) [128]. In addition, the duration of being an agricultural worker was the most influential factor that reducing respiratory function and was more severe in smoking farmers [129].
Table 7.
Herbicide-associated respiratory system disorders
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| Not specifically identified | Human study | - | Significantly associated between high prevalence of respiratory symptoms (wheeze and dyspnea) and farmers who applied high dose and mixed herbicide and ignored wearing PPE. | Sidthilaw et al. [128] |
| Human study | - | Significantly associated with poor pulmonary functions in agricultural workers, especially in prolonged and smoking workers. | Babaoglu et al. [129] | |
| PQ | Rhesus monkeys | 25 to 80 mg/kg | Induced pulmonary fibrosis with collagen deposition and inflammatory cells infiltration. | Shao et al. [130] |
| Human study | - | Increased WISP1 gene expression and developed pulmonary fibrosis in PQ-poisoning cases. | Li et al. [131] | |
| C57BL/6J mice | 40 mg/kg | Induced neutrophil infiltration and lung injury through HBGB1-TLR4-IL23-IL17A axis. | Yan et al. [132] | |
| Sprague-Dawley male rats | 10 and 50 mg/kg | Induced pulmonary fibrosis through Keap1/Nrf2 signaling pathway. | Yang, Xiao, and Shi [133] | |
| C57BL/6J mice | 40 mg/kg | Induced acute lung injury through miR-199-mediated SET. | Cai et al. [134] | |
| Case report | 10 g | Developed asthma attack after successful recovery. | Fan et al. [135] | |
| ATZ | CD1 male mice | 25 mg | Induced pulmonary damage and fibrosis through Nrf2 signaling pathway. | D’Amico et al. [136] |
| CD1 male mice | 250 mg | Induced pulmonary inflammation through the induction of apoptotic Beclin 1/Lc 3 expression and Nrf2 signaling pathway. | D’Amico et al. [137] | |
| 2,4-D | A549 and WI38 cell lines | Maximum at 200 µM | Induced lung cytotoxicity through the disruption of the cellular tubulin-microtubule network. | Ganguli et al. [138] |
Extensive studies have investigated lung toxicity of PQ, as it represents the primary site of action following the uptake [139]. Similar pathological changes seen in acute PQ poisoning cases were also observed after exposure for several weeks [130]. Based on the provided findings [131–134], PQ-induced lung toxicity involves several mechanisms. It increased pro-inflammatory cytokines through the stimulation of high mobility group box 1 (HMGB1) protein and elevated Wnt-induced secreted protein-1 (WISP1) level, a marker of pulmonary fibrosis. Additionally, PQ also disrupted the Keap/Nrf2 signaling pathway, promoting iron accumulation in the lungs, and subsequent lipid peroxidation and ferroptosis. The miRNA-199-mediated decrease in SET protein further contributes to lung injury, resulting in edema and hemorrhage. In addition, some PQ poisoning survivors may experience obstructive ventilatory dysfunction even after full recovery, including symptoms such as coughing and wheezing, suggesting the potential for long-term respiratory complications [135].
Although the evidence for lung toxicity induced by exposure to other herbicide may not be extensive as that for PQ, they have also been associated with lung damage. ATZ and 2,4-D shared similar toxic effects with PQ by inducing oxidative stress, disrupting antioxidant defenses, and promoting cell death processes. Besides the disturbance of antioxidant defense system [136, 137], ATZ and 2,4-D promote cell autophagy by increasing a pro-apoptotic protein BAX as well as decreasing an anti-apoptotic BCL-2 [137, 138]. Furthermore, Ganguli et al. [138] proposed a key mechanism of 2,4-D-induced lung cytotoxicity by targeting the tubulin in A549 and WI38 cell lines. In silico experiment additionally confirmed the stable interaction between 2,4-D and tubulin which might lead to the loss of cell shape, morphology, and ultimately cell shrinkage.
Immunological disorders
Herbicides can disturb the immune system in both transient and permanent alterations, as detailed in Table 8. The incidence of rheumatoid arthritis among farmers was associated with the use of ATZ (OR = 1.62, 95% CI: 1.09–2.40) [140], and GLY (OR = 1.4, 95% CI: 1.0–2.1) [141]. The prolonged use of metribuzin for more than 10 days in a year strongly increased the risk of systemic autoimmune diseases (SLEs) and Sjogren’s syndrome (SS) in older adults and females (HR = 5.33, 95% CI: 2.19–12.96) [142]. Additionally, several herbicide including 2,4-D, GLY, ATZ, and SIM were also significantly related to allergic wheeze in male farmers [143].
Table 8.
Herbicide-associated immunological disorders
| Herbicide | Subjects | Dose Exposure | Main findings | Ref |
|---|---|---|---|---|
| Metribuzin | Human analysis | - | Significantly associated with the risk of systemic autoimmune diseases (SLEs) and Sjogren’s syndrome (SS) in participants who exposed more than ten days a year. | Parks et al. [142] |
| 2,4-D, ATZ, GLY, and SIM | Human analysis | - | Significantly associated with allergic wheeze. | Hoppin et al. [143] |
| PQ | Human analysis | - | Increased neutrophils and decreased lymphocytes levels. | Gao et al. [144] |
| C57BL/6J mice | 20 mg/kg | Induced the apoptosis in effector or activated lymphocytes resulting in impaired memory immune response. | Shao et al. [145] | |
| GLY | Human analysis | - | Significantly increased the risk of rheumatoid arthritis. | Parks et al. [141] |
| Cyprinus carpio | 0.25 to 2 mg/L | Induced immune depression effect and altered other biochemical and hematological parameters. | Yousefi et al. [146] | |
| BALB/c female mice | 87.5 µg to 8.75 mg | F0 exhibited a slight eosinophil in lung lavage and increased TH2 cytokine production. Reduced immune response was observed only in female F1 mice. An abundant gut microbiome was also founded in F1offsping. | Buchenauer et al. [147] | |
| ATZ | Human analysis | - | Significantly associated with rheumatoid arthritis in lifetime days of use applicators. | Meyer et al. [140] |
| BALB/c female mice | 23, 90, and 360 mg/kg | Altered the pathology of immune-related organs and decreased the proliferation ability of lymphocytes. | Chang et al. [148] | |
| Honeybee | 3.73 and 37.3 mg/L | Inhibited the expression of immunity, lipid metabolism, detoxification, and chemosensory genes as well as altered gut microbial composition. | Wang et al. [149] |
Gao et al. [144] investigated the alterations of T lymphocytes and their subgroups (CD4 and CD8) between the survival and fatal PQ poisoning cases. Studies have shown that PQ exposure led to reduced lymphocyte numbers, impaired T-cell function, and reduced antibody production. The reduction in number could result from apoptotic induction of the late-stage effector lymphocyte as reported by Shao et al. [145]. GLY and ATZ have also been linked to immunosuppression [146], suspected that these effects were likely mediated by alterations in gut microbiota [149]. Exposure to ATZ has been shown to reduce spleen weight, splenocyte numbers, and white blood cell counts, as well as impair T and B lymphocytes function [148]. Moreover, Buchenauer et al. [147] studied not only the direct impact of allergic immune response in pure GLY-treated mice but also the influence of its exposure during pregnancy and breastfeeding on the development of asthma in the offspring. Maternal GLY exposure disrupted the immune response in F1 generation offspring, affecting lung inflammation, IgE levels, and cytokine production. The altered responses occurred only in this generation and were speculated to be mediated by microbiota alteration since abundant gut bacteria like Odoribacter and Lachnospiraceae NK4A136 were found [147].
Diminished implementation of herbicide exposure
The comprehensive review of potential health effects related to herbicide exposure encompasses various groups including not only direct applicators but also nearby residents, pregnant women, newborns, and children. The foremost insights from each finding could bring attention to diverse health concerns, emphasizing the need for collective efforts to alleviate their impacts. Providing well-enforced guidelines and good practices on herbicide usage and investing in research to develop safer options (e.g., herbicide substitution, remediation techniques, and enhanced detection methods) are integral components (Fig. 3). Each implementing strategy possesses distinct characteristics, advantages, and limitations in mitigating risks of herbicide-related human health problems, as provided in Table 9.
Fig. 3.
Diminished implementation of herbicide exposure (By author)
Table 9.
Characteristics, advantages, and limitations of diminished implementation of herbicide exposure
| Strategy | Key outputs | Advantages | Limitations |
|---|---|---|---|
| Guidelines and practices |
• Provide a framework for controlling herbicide usage and minimizing exposure risks. • Standardize practices across the country. • Raise awareness among the community regarding safe handling and disposal procedures. |
• Strict adherence to provided guidelines can optimize herbicide use, leading to reduced waste and costs. • Strict adherence to provided guidelines can enhance the credibility and safety of products. |
• Compliance may be challenging to monitor effectively, especially in remote areas. • Enforcement varies based on regulatory infrastructure and resources. |
| Bioherbicides | • Enable to be biodegradable and environmentally friendly. |
• Offer potentially safer and more sustainable alternatives. • Enable to reduce the herbicide resistance. |
• Development and registration processes are time-consuming and expensive. • Effectiveness varies based on many factors such as timing and environmental conditions. |
| Remediation techniques | • Provide options for restoring ecosystems and contaminated areas affected by herbicide pollution. | • Enable to increase the value of contaminated properties by making them suitable for redevelopment or reuse. |
• Effectiveness varies based on the type and extent of contamination. • Certain remediation techniques are costly and impractical for large-scale implementation. |
| Monitoring and detection methods |
• Allow early detection of herbicide contamination in environmental resources and commodities. • Provide real-time monitoring. |
• Enable prompt action to mitigate its effects by timely detect the excessive herbicide residues. • Enable to monitor the effectiveness of remediation efforts and prevent future contamination. |
• Certain detection methods may have limited sensitivity and specificity. • Certain detection methods rely on sophisticated equipment and expertise. |
Well enforce guidelines and good practices
The regulations and policies set by governmental agencies and international organizations can provide the safety precautions, usage restrictions, and alternative weed control measures to achieve effective weed management, as well as promote sustainable agriculture while safeguarding human health. According to the FAO, good agricultural practice (GAP) was introduced to establish the standard for ensuring sustainable and responsible management in agricultural practices [150, 151]. The critical suggestions regarding health typically include (i) the proper way to handle agrichemicals like pesticides and fertilizers involving the cautious selection of appropriate chemicals for specific pests and strictly following the legal instructions; (ii) the practice of keeping productive areas away from the hazardous contamination such as avoid performing agrichemicals near water sources; (iii) the effective waste management; and (iv) well-trained programs for workers to increase self-literacy in handling the dangerous materials. Moreover, GAP certification enables numerous advantages for farmers and agricultural producers, ultimately benefiting consumers. GAP certification is often a prerequisite for import/export operations to global commerce, which also expands the market size opportunity and ensures compliance with international trade regulations as required by many countries and retailers [150]. Thus, this certification has fostered competitive products by encouraging the producers to participate in environmentally responsible practices through efficient resource utilization, chemical input reduction, proper waste, and risk management. Furthermore, the integrated weed management (IWM) approach is growing in popularity since it aligns with sustainable and environmentally friendly practices. IWM provides various aspects for weed control that enable to lessen the chemical inputs, reducing the interconnectedness between herbicide remaining and human health. The whole system for this alternative weed management includes four basic strategies which are physical (i.e., hoeing and flaming), biological (i.e., natural enemy utilization), ecological (i.e., crop rotation), and chemical (i.e., proper herbicide selection) management [152]. Although the practices mentioned above predominately focus on herbicide usage in agricultural contexts, its key details could be extrapolated to ensure effective applications in other more minor environmental scales such as private lawns and gardens.
To establish the internationally acceptable residue level that facilitates the standard for global trade harmonization, and ensure food safety in compliance with GAP, the Joint Meeting on Pesticides Residues (JMPR) conducts scientific assessments of pesticide residues in agricultural commodities [153]. Subsequently, the Codex Maximum Residue Level (MRL) has been published regarding the highest pesticides residue level that food or feed can legally contain [154]. In addition, the pesticidal MRLs could be independently determined by the national regulatory agencies sharing the same goal of ensuring the safety of domestically produced and imported products. Examples of national regulatory agencies responsible for, but not limited to, enforcing pesticides MRLs include the Pest Management Regulatory Agency (PMRA) of Canada, the Australian Pesticides and Veterinary Medicines Authority (APVMA) of Australia, and the Environmental Protection Agency (EPA) of the United States [155]. Individual regulatory agencies also hold the authority to take decisive actions such as imposing restrictions or outright bans on highly hazardous agrichemicals when the scientific evidence demonstrates risks to human health. For instance, Thailand’s National Hazardous Substance Committee (NHSC) has initiated a review of the safety of PQ, GLY, and chlorpyrifos in 2017. Based on the available scientific literature, the NHSC decided to ban PQ and chlorpyrifos within the country, effective June 1, 2020, whereas GLY remains subject to restrictions for domestic use [156].
Bioherbicides
Bioherbicides (also known as allelopathic or allelochemical compounds) have emerged as a promising method by applying the potential of natural compounds for weed control [157]. They can inhibit the weed growth by releasing toxic metabolites or disrupting regular functions [158]. There are mainly two groups based on the different sources: plant-based and microbial-based herbicides. Plant-based herbicides, such as extracts from Cynara cardunculus, have shown effectiveness in controlling various weeds by potentially inhibiting seed growth and germination, leading to necrosis and chlorosis [159]. The performance was also comparable to commercial bioherbicides containing pelargonic acid. Microbial-based herbicides, like toxins produced by Phoma sp., can also be effective when formulated with appropriate carriers. Target plants showed severe symptoms after spraying the formulation (e.g., yellowish appearance on leaves, growth retardation, and necrosis) [160]. In addition, several bioherbicides have already been launched in global markets or held patents such as BioWeed™, Organic interceptor®, Katoun Gold®, and Matratec® [157]. Nonetheless, several limitations currently impede the broader adoption of bioherbicides in agricultural practices. To fully reallize the potential of bioherbicides in sustainable weed management, future advancements should prioritize environmental stability, selectivity, absorption efficiency, and cost-effectiveness [157].
Remediation techniques
Implementing removal strategies, including microbial remediation, natural adsorbents, and electrochemical advanced oxidation processes (EAOPs), is a practical approach to reduce the accumulation of the residues in environmental resources. Microbial remediation, mainly derived from bacteria and fungi, is considered an environmentally harmless, cost-effective, and highly efficient technique. It can break down molecules into less toxic byproducts which further serve as the source of energy, carbon, and minerals [161]. For instance, Mohanty and Jena [162] used an isolated Pseudomonas putida to degrade butachlor. This strain could effectively remediate 700 mg/L of butachlor within 360 h. and its remediation efficiency was further enhanced by immobilizing on calcium alginate beads. Zhu et al. [163] identified Bacillus altitudinis as a fast SIM bio-degrader, with a potency enhanced by bioaugmentation with Pennisetum rhizosphere soil. This provided additional enzymes or factors that promoted catalytic activity, resulting in 97% degradation of 30 mg/kg SIM within 5 days.
EAOPs and absorbent techniques are additional options for herbicide removal. EAOPs use electric current to generate ROS that oxidize and degrade organic contaminants into carbon dioxide, water, or less harmful metabolites [164]. This technique offers the independence of auxiliary chemical reagents or the catalyst in the system and applies to both soil and water treatment [164, 165]. On the other hand, absorption strategies use environmentally friendly materials like activated carbon, clay minerals, or agricultural waste, to absorb or remove toxic residues from natural resources [166]. This technique offers simplicity, user-friendliness, versatility, and high efficiency.
Monitoring and detection methods
It is widely acknowledged that conventional detection methods such as chromatography-based techniques could offer high sensitivity and specificity toward complex sample matrix analysis. Nevertheless, these methods are hindered by time-consumed analysis, extensive sample preparation steps, costly instrument setup, and the need for expertise, limiting their widespread adoption, especially in resource-limited settings or among non-expert users. The advancement of residue detection methods has then concurrently gained attention since chemical-based herbicide is unlikely to be suspended in the near future. It is essential to enhance the efficiency of detection methods by considering factors such as time, cost, portability, and ease of use to prevent the excessive accumulation of natural resources, the contamination of edible commodities, or even their application in clinical settings. Electrochemical, surface-enhanced Raman scattering (SERS), and artificial intelligence (AI) techniques are examples of detection methods that pose favorable properties to meet these criteria [167]. Interestingly, computational power is believed to provide innovative solutions and improve agricultural practices, whether by automated weed detection or robotic spraying vehicles [168]. However, all these promising options still require development and testing such as for reliability, stability, and feasibility testing under real-world scenarios before authorization.
Conclusion and future prospective
The substantial utilization of herbicide conceivably causes various health implications. These chemicals can induce threats at any life stage and affect most biological systems through multiple pathways whether by inducing oxidative stress or directly disrupting processes at both molecular and cellular levels. The potential adverse effects on human health should remain a concern, despite some scientific evidence demonstrating conflict outcomes. The main findings emphasize the urgent need for concerted efforts to reduce these impacts on public health. Individuals, communities, and national regulatory bodies are all responsible for fostering a safer society. This may include strengthening health awareness, developing better practices and technologies, as well as improving monitoring and surveillance systems. Furthermore, future research is urged to explore the relationship between human health and herbicide exposure, as certain aspects remain inadequately studied. Conducting such studies is crucial for facilitating early decision-making concerning agricultural practices, regulatory policies, and public health measures.
Acknowledgements
Not applicable.
Authors’ contributions
Conceptualization: [K.P., T.T.]; Methodology: [K.P., T.T., W.R.]; Formal analysis: [J.H., F.A., W.R.]; Investigation [J.H., F.A.]: Funding acquisition: [K.P., J.H.]; Resources: [K.P., T.T.]; Writing - original draft preparation: [J.H., F.A.]; Writing - review and editing: [K.P., T.T., W.R.]; Supervision: [K.P.]
Funding
This work was supported by Mahidol University (Scholarships for Ph.D. student 2022); and partially supported by Faculty of Graduate Studies, Mahidol University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. World population projected to reach 9.8 billion in 2050, and 11.2 billion in 2100. 2017. https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100.
- 2.Reddy PP. Impacts on Weeds. In: Climate Resilient Agriculture for Ensuring Food Security [Internet]. New Delhi: Springer India; 2015. p. 193–205. [cited 2024 Apr 11]. Available from: https://link.springer.com/10.1007/978-81-322-2199-9_10.
- 3.NCBI. History of the Controversy Over the Use of Herbicides [Internet]. 1994. Available from: https://www.ncbi.nlm.nih.gov/books/NBK236351/#.
- 4.Stallings KD, Seth-Carley D, Richardson RJ. Management of aquatic vegetation in the Southeastern United States. J Integr Pest Manage. 2015;6(1):3–3. [Google Scholar]
- 5.Wagner V, Antunes PM, Irvine M, Nelson CR. Herbicide usage for invasive non-native plant management in wildland areas of North America. J Appl Ecol. 2017;54(1):198–204. [Google Scholar]
- 6.Rojas J, Dhar A, Naeth M. Urban naturalization for green spaces using soil tillage, herbicide application, compost amendment and native vegetation. Land. 2021;10(8):854. [Google Scholar]
- 7.Statista. Global Pesticide Agricultural Use 2021, by Type [Internet]. 2023. Available from: https://www.statista.com/statistics/1263206/global-pesticide-use-by-type/.
- 8.Schleiffer M, Speiser B. Presence of pesticides in the environment, transition into organic food, and implications for quality assurance along the European organic food chain – A review. Environ Pollut. 2022;313:120116. [DOI] [PubMed]
- 9.Paola Balderrama-Carmona A, Patricia Silva-Beltrán N, Alberto Zamora Alvarez L, Patricia Adan Bante N, Felipe Moran Palacio E. Consequences of Herbicide Use in Rural Environments and Their Effect on Agricultural Workers. In: Sustainability Concept In Developing Countries [Working Title] [Internet]. IntechOpen; 2020. [cited 2024 Apr 11]. Available from: https://www.intechopen.com/online-first/consequences-of-herbicide-use-in-rural-environments-and-their-effect-on-agricultural-workers.
- 10.Gawarammana IB, Buckley NA. Medical management of Paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thunga G, Vijaynarayana K, Sreedharan N, Varma M, Pandit V, Cherukuri H, et al. Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicol Int. 2014;21(2):209. [DOI] [PMC free article] [PubMed]
- 12.FAOSTAT. Pesticides Use [Internet]. n.d. Available from: https://www.fao.org/faostat/en/#data/RP/visualize.
- 13.Observatory of Economic Complexity. Brazil [Internet]. n.d. Available from: https://oec.world/en/profile/country/bra.
- 14.Alcántara-de La Cruz R, Moraes De Oliveira G, Bianco De Carvalho L, Fátima Das Graças Fernandes Da Silva M. Herbicide Resistance in Brazil: Status, Impacts, and Future Challenges. In: Kontogiannatos D, Kourti A, Ferreira Mendes K, editors. Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production [Internet]. IntechOpen; 2020. [cited 2024 Apr 11]. Available from: https://www.intechopen.com/books/pests-weeds-and-diseases-in-agricultural-crop-and-animal-husbandry-production/herbicide-resistance-in-brazil-status-impacts-and-future-challenges.
- 15.Center for Food Safety (CFS). Pesticides | Glyphosate [Internet]. 2022. Available from: https://www.centerforfoodsafety.org/issues/6459/pesticides/glyphosate.
- 16.Center for Food Safety (CFS). Pesticides | 2,4-Dichlorophenoxyacetic Acid (2,4-D) [Internet]. n.d. Available from: https://www.centerforfoodsafety.org/issues/6459/pesticides/24-d.
- 17.Center for Food Safety (CFS). Pesticides | Atrazine [Internet]. n.d. Available from: https://www.centerforfoodsafety.org/issues/6459/pesticides/atrazine.
- 18.U.S. Geological Survey. Estimated Annual Agricultural Pesticide Use [Internet]. 2024. Available from: https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2019&map=PARAQUAT&hilo=L.
- 19.REACH24H Consulting Group - Value In Compliance. Analysis of Herbicide Registration in China: Statistics in 2015-2022, Part 1/2 -REACH24H [Internet]. 2022. Available from: https://www.reach24h.com/en/news/industry-news/agrochemical/analysis-of-herbicides-registration-in-china-statistics-in-2015-2022-part-1-2.html.
- 20.Casimero M, Abit MJ, Ramirez AH, Dimaano NG, Mendoza J. Herbicide use history and weed management in Southeast Asia. Advances in weed science. 2022;40(spe1):e020220054. 10.51694/AdvWeedSci/2022;40:seventy-five013. [Google Scholar]
- 21.Kwonpongsagoon S, Katasila C, Kongtip P, Woskie S. Application intensity and spatial distribution of three major herbicides from agricultural and nonagricultural practices in the central plain of Thailand. IJERPH. 2021;18(6):3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eurostat. EU sales of pesticides rebounded in 2021. 2023. https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20230510-1.
- 23.Tudi M, Daniel Ruan H, Wang L, Lyu J, Sadler R, Connell D, et al. Agriculture Development, Pesticide Application and Its Impact on the Environment. IJERPH. 2021;18(3):1112. 10.3390/ijerph18031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fishel FM. Pesticides: Routes of Exposure [Internet]. 2015. Available from: https://journals.flvc.org/edis/article/download/127676/128025/212288.
- 25.Sarwar M. The Dangers of Pesticides Associated With Public Health and Preventing of the Risks [Internet]. n.d. Available from: http://files.aiscience.org/journal/article/pdf/7007.
- 26.Gold V, editor. The IUPAC Compendium of Chemical Terminology: The Gold Book [Internet]. 4th ed. Research Triangle Park, NC: International Union of Pure and Applied Chemistry (IUPAC); 2019 [cited 2024 Apr 11]. Available from: https://goldbook.iupac.org/.
- 27.ChemSafetyPro. GHS Classification Criteria for Acute Toxicity [Internet]. Available from: https://www.chemsafetypro.com/Topics/GHS/.
- 28.NCBI. Glyphosate [Internet]. n.d. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Glyphosate.
- 29.NCBI. Atrazine [Internet]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Atrazine.
- 30.NCBI. 2,4-Dichlorophenoxyacetic acid [Internet]. n.d. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/2_4-Dichlorophenoxyacetic-acid.
- 31.NCBI. Paraquat. n.d.c. https://pubchem.ncbi.nlm.nih.gov/compound/Paraquat.
- 32.CDC. Facts about paraquat [Internet]. n.d. Available from: https://emergency.cdc.gov/agent/paraquat/basics/facts.asp.
- 33.WHO. Suicide [Internet]. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/suicide.
- 34.Lee JW, Hwang IW, Kim JW, Moon HJ, Kim KH, Park S, et al. Common Pesticides Used in Suicide Attempts Following the 2012 Paraquat Ban in Korea. J Korean Med Sci. 2015;30(10):1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutkule DP, Venkategowda PM, Mahendrakar K. Glyphosate surfactant herbicide poisoning and management. Indian J Crit Care Med. 2014;18(5):328–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang C-B, Chia-Chu C. Refractory cardiopulmonary failure after glyphosate surfactant intoxication: a case report. J Occup Med Toxicol. 2009;4(1):2. 10.1186/1745-6673-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picetti E, Generali M, Francesca M, Giampaolo N, Roberta D, Giuseppe L, et al. Glyphosate ingestion causing multiple organ failure: a near-fatal case report. 2017. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6166172/. [DOI] [PMC free article] [PubMed]
- 38.Kunapareddy T, Kalisetty S. Glyphosate poisoning – a Case Report. J Postgrad Med. 2021;67(1):36–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khot R, Joshi P, Pandharipande M, Nagpure K, Thakur D. Glyphosate poisoning with acute pulmonary edema. Toxicol Int. 2014;21(3):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janeela MA, Oommen A, Misra A, Ramya I. Paraquat poisoning: case report of a survivor. J Family Med Prim Care. 2017;6(3):672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh S, Ghosh S, Dewan H, Walia G. Herbicide poisoning: A diagnostic challenge. Indian J Crit Care Med. 2012;16(1):52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigdel KR, Bhattarai S, Thapa A, Dahal A, Adhikari S, Panthi RC, et al. Case Report: Paraquat poisoning. Wellcome Open Res. 2022;7:171. [Google Scholar]
- 43.Yu G, Kan B, Jian X, Wang J, Sun J, Song C. A case report of acute severe paraquat poisoning and long-term follow-up. Exp Ther Med. 2014;8(1):233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Q, Kan B, Jian X, Zhang W, Liu H, Zhang Z. Paraquat poisoning by skin absorption: two case reports and a literature review. Exp Ther Med. 2013;6(6):1504–6. 10.3892/etm.2013.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiran S, Kumar S. 2, 4-D dichlorophenoxyacetic acid poisoning; case report and literature review. Asia Pac J Med Toxicol. 2017;6(1):29–33. [Google Scholar]
- 46.Demissie Z, Bekele A, Bane A. A case of severe 2,4-Dichlorophenoxyacetic acid poisoning causing diagnostic and treatment challenges. IMCRJ. 2022;15:389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Gupta S, Bansal YS, Bal A, Rastogi P, Muthu V, et al. Pulmonary histopathology in fatal paraquat poisoning. Autops Case Rep. 2021;11:e2021342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clinical Management of Acute Pesticide Intoxication. The management of pesticide poisoned patients at various levels of health care. 2008. https://www.ncbi.nlm.nih.gov/books/NBK185112/.
- 49.US EPA. Using Toxicity Tests in Ecological Risk Assessment [Internet]. 1994. Available from: https://www.epa.gov/sites/default/files/2015-09/documents/v2no1.pdf.
- 50.Mohammad Ahmadi Soleimani S, Ekhtiari H, Cadet JL. Drug-induced neurotoxicity in addiction medicine. In: Progress in Brain Research [Internet]. Elsevier; 2016. p. 19–41. [cited 2024 Apr 11]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079612315001107. [DOI] [PubMed] [Google Scholar]
- 51.Manager, Ilo Content. Chemical Neurotoxic Agents [Internet]. 2011. Available from: https://www.iloencyclopaedia.org/part-i-47946/nervous-system/item/289-chemical-neurotoxic-agents.
- 52.Narayan S, Liew Z, Bronstein JM, Ritz B. Occupational pesticide use and parkinson’s disease in the Parkinson Environment Gene (PEG) study. Environ Int. 2017;107:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayden KM, Norton MC, Darcey D, Østbye T, Zandi PP, Breitner JCS, et al. Occupational exposure to pesticides increases the risk of incident AD: The Cache County Study. Neurology. 2010;74(19):1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adani G, Filippini T, Garuti C, Malavolti M, Vinceti G, Zamboni G, et al. Environmental Risk Factors for Early-Onset Alzheimer’s Dementia and Frontotemporal Dementia: A Case-Control Study in Northern Italy. IJERPH. 2020;17(21):7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan D, Zhang Y, Liu L, Yan H. Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep. 2016;6(1):32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Luca G, Russo MT, Degan P, Tiveron C, Zijno A, Meccia E, et al. A role for oxidized DNA precursors in Huntington’s disease–like Striatal Neurodegeneration. Orr H, editor. PLoS Genet. 2008;4(11):e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong T, Duan W, Xu Y, Hong H, Xu J, Fu G, et al. Paraquat exposure induces Parkinsonism by altering lipid profile and evoking neuroinflammation in the midbrain. Environ Int. 2022;169:107512. [DOI] [PubMed] [Google Scholar]
- 58.Pinho BR, Reis SD, Hartley RC, Murphy MP, Oliveira JMA. Mitochondrial superoxide generation induces a parkinsonian phenotype in zebrafish and huntingtin aggregation in human cells. Free Radic Biol Med. 2019;130:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winstone JK, Pathak KV, Winslow W, Piras IS, White J, Sharma R, Huentelman MJ, Pirrotte P. Glyphosate infiltrates the brain and increases proinflammatory cytokine TNFα: implications for neurodegenerative disorders. J Neuroinflamm. 2022;19(1):193. 10.1186/s12974-022-02544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez A, Al-Ahmad AJ. Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicol Lett. 2019;304:39–49. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Qi L, Chen Y, Lv H, Bi H. Bioinformatics analysis of the potential mechanisms of Alzheimer’s disease induced by exposure to combined triazine herbicides. Ann Hum Biol. 2023;50(1):442–51. [DOI] [PubMed] [Google Scholar]
- 62.Aloizou AM, Siokas V, Vogiatzi C, Peristeri E, Docea AO, Petrakis D, et al. Pesticides, cognitive functions and dementia: A review. Toxicol Lett. 2020;326:31–51. [DOI] [PubMed] [Google Scholar]
- 63.Dich J, Hoar Zahm S, Hanberg A, Adami HO. Pesticides and Cancer on JSTOR [Internet]. 1997. Available from: http://www.jstor.org/stable/3552701. [DOI] [PubMed]
- 64.Shah SC, Boffetta P, Johnson KC, Hu J, Palli D, Ferraroni M, et al. Occupational exposures and odds of gastric cancer: a StoP project consortium pooled analysis. Int J Epidemiol. 2020;49(2):422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han MA, Kim JH, Song HS. Persistent organic pollutants, pesticides, and the risk of thyroid cancer: systematic review and meta-analysis. Eur J Cancer Prev. 2019;28(4):344–9. [DOI] [PubMed] [Google Scholar]
- 66.Koutros S, Silverman DT, Alavanja MC, Andreotti G, Lerro CC, Heltshe S, et al. Occupational exposure to pesticides and bladder cancer risk. Int J Epidemiol. 2016;45(3):792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peñalver-Piñol A, Benavente Y, Frias-Gomez J, Alguacil J, Santibañez M, Contreras-Llanes M, et al. Occupational exposure to pesticides and endometrial cancer in the Screenwide casecontrol study. Environ Health. 2023;22(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lerro CC, Koutros S, Andreotti G, Hines CJ, Blair A, Lubin J, et al. Use of acetochlor and cancer incidence in the Agricultural Health Study. Int J Cancer. 2015;137(5):1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onyije FM, Dolatkhah R, Olsson A, Bouaoun L, Deltour I, Erdmann F, et al. Risk factors for childhood brain tumours: a systematic review and meta-analysis of observational studies from 1976 to 2022. Cancer Epidemiol. 2024;88:102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, et al. Cancer Incidence among Glyphosate-Exposed Pesticide Applicators in the Agricultural Health Study. Environ Health Perspect. 2005;113(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meloni F, Satta G, Padoan M, Montagna A, Pilia I, Argiolas A, et al. Occupational exposure to glyphosate and risk of lymphoma:results of an Italian multicenter case-control study. Environ Health. 2021;20(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang VC, Zhou W, Berndt SI, Andreotti G, Yeager M, Parks CG, et al. Glyphosate use and Mosaic loss of chromosome Y among male farmers in the agricultural health study. Environ Health Perspect. 2023;131(12):127006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahmoudinia S, Niapour A, Ghasemi Hamidabadi H, Mazani M. 2,4-D causes oxidative stress induction and apoptosis in human dental pulp stem cells (hDPSCs). Environ Sci Pollut Res. 2019;26(25):26170–83. [DOI] [PubMed] [Google Scholar]
- 74.Mariotti VCBS, Naufal IZF, Amorim IAR, Parizi JLS, Nai GA. Digestive tract toxicity associated with exposure to 2,4-dichlorophenoxyacetic acid in rats. Braz J Med Biol Res. 2022;55:e12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wafa T, Amel N, Issam C, Imed C, Abdelhedi M, Mohamed H. Subacute effects of 2,4-dichlorophenoxyacetic herbicide on antioxidant defense system and lipid peroxidation in rat erythrocytes. Pestic Biochem Physiol. 2011;99(3):256–64. [Google Scholar]
- 76.Remigio RV, Andreotti G, Sandler DP, Erickson PA, Koutros S, Albert PS, et al. An updated evaluation of Atrazine-Cancer Incidence associations among pesticide applicators in the Agricultural Health Study Cohort. Environ Health Perspect. 2024;132(2):027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SK, Kang D, Beane-Freeman L, Blair A, Hoppin JA, Sandler DP, et al. Cancer incidence among paraquat exposed applicators in the Agricultural Health Study: a prospective cohort study. Int J Occup Environ Health. 2009;15(3):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alizadeh S, Anani-sarab G, Amiri H, Hashemi M. Paraquat induced oxidative stress, DNA damage, and cytotoxicity in lymphocytes. Heliyon. 2022;8(7):e09895. [DOI] [PMC free article] [PubMed]
- 79.Petrovská H, Dušinská M. Oxidative DNA damage in human cells induced by Paraquat. Altern Lab Anim. 1999;27(3):387–95. [DOI] [PubMed]
- 80.Andreotti G, Koutros S, Hofmann JN, Sandler DP, Lubin JH, Lynch CF, et al. Glyphosate Use and Cancer Incidence in the Agricultural Health Study. JNCI: J Nat Cancer Inst. 2018;110(5):509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.US EPA. Glyphosate [Internet]. 2023. Available from: https://www.epa.gov/ingredients-used-pesticide-products/glyphosate.
- 82.WHO. IARC Monograph on Glyphosate [Internet]. 2018. Available from: https://www.iarc.who.int/featured-news/media-centre-iarc-news-glyphosate/.
- 83.Bus JS. IARC use of oxidative stress as key mode of action characteristic for facilitating cancer classification: Glyphosate case example illustrating a lack of robustness in interpretative implementation. Regul Toxicol Pharmacol. 2017;86:157–66. [DOI] [PubMed] [Google Scholar]
- 84.IARC. IARC Monographs Evaluate DDT, Lindane, and 2,4-D [Internet]. 2015. Available from: https://www.iarc.who.int/wp-content/uploads/2018/07/pr236_E.pdf.
- 85.Burns C, Bodner K, Swaen G, Collins J, Beard K, Lee M. Cancer Incidence of 2,4-D Production Workers. IJERPH. 2011;8(9):3579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang D, Wu Y, Yuan Y, Liu W, Kuang H, Yang J, et al. Exposure to 2,4-dichlorophenoxyacetic acid induces oxidative stress and apoptosis in mouse testis. Pestic Biochem Physiol. 2017;141:18–22. [DOI] [PubMed] [Google Scholar]
- 87.Pochettino AA, Bongiovanni B, Duffard RO, Evangelista De Duffard AM. Oxidative stress in ventral prostate, ovary, and breast by 2,4‐dichlorophenoxyacetic acid in pre‐ and postnatal exposed rats. Environ Toxicol. 2013;28(1):1–10. [DOI] [PubMed]
- 88.ATSDR. ToxFAQsTM for Atrazine [Internet]. 2011. Available from: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=854&toxid=59.
- 89.Onur B, Çavuşoğlu K, Yalçin E, Acar A. Paraquat toxicity in different cell types of Swiss albino mice. Sci Rep. 2022;12(1):4818. [DOI] [PMC free article] [PubMed]
- 90.Laws MJ, Neff AM, Brehm E, Warner GR, Flaws JA. Endocrine disrupting chemicals and reproductive disorders in women, men, and animal models. In: Advances in Pharmacology [Internet]. Elsevier; 2021. p. 151–90. [cited 2024 Apr 11]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054358921000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cragin LA, Kesner JS, Bachand AM, Barr DB, Meadows JW, Krieg EF. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking Water. Environ Res. 2011;111(8):1293–301. 10.1016/j.envres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Nassar SE, et al. Effects of herbicide atrazine on reproductive hormonal levels, Cytochrome P450, and gonadal structure of adult male and female crayfish, Procambarus clarkii. Egypt J Aquat Biol Fish. 2022;26(6):1095–114.
- 93.Fa S, Pogrmic-Majkic K, Samardzija D, Glisic B, Kaisarevic S, Kovacevic R. Involvement of ERK1/2 signaling pathway in atrazine action on FSH-Stimulated LHR and CYP19A1 expression in rat granulosa cells. Toxicol Appl Pharmcol. 2013;270(1):1–8. [DOI] [PubMed]
- 94.Foradori CD, Prägati S, Coder M, Tisdel KD, Yi JW, Simpkins RJ, Handa. The effect of atrazine administered by gavage or in diet on the LH Surge and reproductive performance in intact female Sprague-Dawley and Long Evans rats. Birth Defects Res Part B: Dev Reproductive Toxicol. 2014;101(3):262–75. [DOI] [PMC free article] [PubMed]
- 95.Rotimi DE, Oluwafemi A, Ojo, Adeyemi OS. Atrazine exposure caused oxidative stress in male rats and inhibited brain-pituitary‐testicular functions. J Biochem Mol Toxicol. 2024;38(1):e23579. [DOI] [PubMed]
- 96.Tinfo NS, Hotchkiss MG, Buckalew AR, Zorrilla LM, Cooper RL, Laws SC. Understanding the effects of atrazine on steroidogenesis in rat granulosa and H295R adrenal cortical carcinoma cells. Reprod Toxicol. 2011;31(2):184–93. [DOI] [PubMed]
- 97.Holloway AC, Anger DA, Crankshaw DJ, Wu M, Foster WG. Atrazine-induced changes in aromatase activity in Estrogen Sensitive Target tissues. J Appl Toxicol. 2008;28(3):260–70. [DOI] [PubMed]
- 98.Harris SM, Jin Y, Loch-Caruso R, Padilla IY, Meeker JD. Identification of environmental chemicals targeting miscarriage genes and pathways using the comparative toxicogenomics database. Environ Res. 2020;184:109259. [DOI] [PMC free article] [PubMed]
- 99.Marouani N, Tebourbi O, Cherif D, Hallegue D, Yacoubi MT, Sakly M, Benkhalifa M, Ben Rhouma K. Effects of oral administration of 2,4-Dichlorophenoxyacetic acid (2,4-D) on reproductive parameters in male Wistar rats. Environ Sci Pollut Res. 2017;24(1):519–26. [DOI] [PubMed]
- 100.Li H, Zhu Q, Wang S, Huang T, Li X, Ni C, Fang Y, Li L, Lian Q, Ge RS. Paraquat exposure delays Stem/Progenitor leydig cell regeneration in the adult rat testis. Chemosphere. 2019;231:60–71. [DOI] [PubMed]
- 101.Sun YL, Wang XL, Yang LL, Ge ZJ, Zhao Y, Luo SM, Shen W, Sun QY. Paraquat reduces the female fertility by impairing the oocyte maturation in mice. Front Cell Dev Biol. 2021;8:631104. [DOI] [PMC free article] [PubMed]
- 102.U.S. Department of Health and Human Services. Toxicological profile for atrazine. [Internet]. 2003. Available from: https://www.atsdr.cdc.gov/toxprofiles/tp153.pdf.
- 103.Wirbisky S. Atrazine exposure and reproductive dysfunction through the hypothalamus-pituitary-gonadal (HPG) Axis. Toxics. 2015;3(4):414–50. [DOI] [PMC free article] [PubMed]
- 104.Goodman M, Mandel JS, DeSesso JM. Atrazine and pregnancy outcomes: a systematic review of epidemiologic evidence. Birth Defects Res Part B: Dev Reprod Toxicol. 2014;101(3):215–36. [DOI] [PMC free article] [PubMed]
- 105.Milesi MM, Lorenz V, Durando M, Rossetti MF, Varayoud J. Glyphosate herbicide: reproductive outcomes and multigenerational effects. Front Endocrinol. 2021;12:672532. [DOI] [PMC free article] [PubMed]
- 106.Brent RL, Beckman DA. Environmental teratogens. 1990. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1809745/pdf/bullnyacadmed00013-0005.pdf. [PMC free article] [PubMed]
- 107.FAO. ATRAZINE. 2005. https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report07/Atrazine.pdf.
- 108.Waller SA, Paul K, Peterson SE, Hitti JE. Agricultural-related chemical exposures, season of conception, and risk of gastroschisis in Washington State. Am J Obstet Gynecol. 2010;202(3):241.e1–241.e6. [DOI] [PubMed]
- 109.Agopian AJ, Cai Y, Langlois PH, Canfield MA, Lupo PJ. Maternal residential atrazine exposure and risk for Choanal Atresia and stenosis in offspring. J Pediatr. 2013;162(3):581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee LJ, Canfield MA, Hashmi SS, Moffitt KB, Marengo L, Agopian AJ, Belmont JW, et al. Association between thyroxine levels at birth and choanal atresia or stenosis among infants in Texas, 2004–2007. Birth Defects Res Part A: Clin Mol Teratol. 2012;94(11):951–4. [DOI] [PubMed] [Google Scholar]
- 111.Galoppo GH, Tavalieri YE, Schierano-Marotti G, Osti MR, Luque EH, Muñoz-de-Toro MM. Long-Term effects of in Ovo exposure to an environmentally relevant dose of atrazine on the thyroid gland of Caiman Latirostris. Environ Res. 2020;186(July):109410. [DOI] [PubMed] [Google Scholar]
- 112.Silver MK, Shao J, Li M, Ji C, Chen M, Xia Y, Lozoff B. Prenatal exposure to the herbicide 2,4-D is associated with deficits in auditory processing during infancy. Environ Res. 2019;172(May):486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Viriato C, Franca FM, Santos DS, Marcantonio AS, Badaro-Pedroso C, Ferreira CM. Evaluation of the potential teratogenic and toxic effect of the herbicide 2,4-D (DMA® 806) in Bullfrog embryos and tadpoles (Lithobates Catesbeianus). Chemosphere. 2021;266(March):129018. [DOI] [PubMed] [Google Scholar]
- 114.Aronzon CM, Sandoval MT, Herkovits J, Pérez-Coll CS. Stage‐dependent toxicity of 2,4‐dichlorophenoxyacetic on the embryonic development of a South American Toad, Rhinella Arenarum. Environ Toxicol. 2011;26(4):373–81. 10.1002/tox.20564. [DOI] [PubMed] [Google Scholar]
- 115.Kongtip P, Nankongnab N, Phupancharoensuk R, Palarach C, Sujirarat D, Sangprasert S, Sermsuk M, Sawattrakool N, Woskie SR. Glyphosate and Paraquat in maternal and fetal serums in Thai women. J Agromed. 2017;22(3):282–9. 10.1080/1059924X.2017.1319315. [DOI] [PubMed] [Google Scholar]
- 116.Pu Y, Yang J, Chang L, Qu Y, Wang S, Zhang K, Xiong Z, et al. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. FASEB J. 2020;34(S1):1–1. 10.1096/fasebj.2020.34.s1.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei D, Wang L, Xu Q, Wang J, Shi J, Ma C, Geng J, et al. Exposure to herbicides mixtures in relation to type 2 diabetes mellitus among Chinese Rural Population: results from different statistical models. Ecotoxicol Environ Saf. 2023;261(August):115109. 10.1016/j.ecoenv.2023.115109. [DOI] [PubMed] [Google Scholar]
- 118.Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, Alavanja MCR. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care. 2007;30(3):529–34. 10.2337/dc06-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Starling AP, David M, Umbach F, Kamel S, Dale L, Sandler P, Jane AH. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med. 2014;71(9):629–35. 10.1136/oemed-2013-101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prasad M, Gatasheh MK, Alshuniaber MA, Krishnamoorthy R, Rajagopal P, Krishnamoorthy K, Periyasamy V, Veeraraghavan VP. Impact of glyphosate on the development of insulin resistance in experimental diabetic rats: role of NFκB Signalling pathways. Antioxidants. 2022;11(12):2436. 10.3390/antiox11122436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jayaraman S, Krishnamoorthy K, Prasad M, Veeraraghavan VP, Krishnamoorthy R, Alshuniaber MA, Gatasheh MK, Elrobh M. Glyphosate potentiates insulin resistance in skeletal muscle through the modulation of IRS-1/PI3K/Akt mediated mechanisms: an in vivo and in silico analysis. Int J Biol Macromol. 2023;242(July):124917. 10.1016/j.ijbiomac.2023.124917. [DOI] [PubMed] [Google Scholar]
- 122.Gupta H, Pawankumar RR, Jha H, Ahmad DK, Patel. Xenobiotic mediated diabetogenesis: developmental exposure to Dichlorvos or Atrazine leads to type 1 or type 2 diabetes in Drosophila. Free Radic Biol Med. 2019;141(September):461–74. 10.1016/j.freeradbiomed.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 123.Lim S, Ahn SY, Song IC, Chung MH, Jang HC, Park KS, Lee KU, Pak YK, Lee HK. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. Edited by German Malaga. PLoS ONE. 2009;4(4):e5186. 10.1371/journal.pone.0005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kimura K, Katsumata Y, Ozawa T, Tawara S, Igarashi K, Cho Y, Shibata N, Hakuno F, Takahashi SI, Takenaka A. Effect of Paraquat-Induced oxidative stress on insulin regulation of insulin-like growth factor-binding Protein-1 gene expression. J Clin Biochem Nutr. 2010;46(2):157–67. 10.3164/jcbn.09-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shibata M, Hakuno F, Yamanaka D, Okajima H, Fukushima T, Hasegawa T, Ogata T, et al. Paraquat-Induced oxidative stress represses phosphatidylinositol 3-Kinase activities leading to impaired glucose uptake in 3T3-L1 adipocytes. J Biol Chem. 2010;285(27):20915–25. 10.1074/jbc.M110.126482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mikov I, Vasović V, Mikov A, Goločorbin-Kon S, Stankov K, Mikov M. Hypoglycemic effect of herbicide 2,4-Dichlorophenoxyacetic acid (2,4-D). Pestic Fitomed. 2010;25(4):349–52. 10.2298/PIF1004349M. [Google Scholar]
- 127.Sun H, Shao W, Liu H, Jiang Z. Exposure to 2,4-Dichlorophenoxyacetic acid induced PPARβ-Dependent disruption of glucose metabolism in HepG2 cells. Environ Sci Pollut Res. 2018;25(17):17050–7. 10.1007/s11356-018-1921-6. [DOI] [PubMed] [Google Scholar]
- 128.Sidthilaw S, Sapbamrer R, Pothirat C, Wunnapuk K, Khacha-Ananda S. Factors associated with respiratory symptoms among herbicide applicators and assistant applicators in maize field. Arch Environ Occup Health. 2022;77(4):320–7. 10.1080/19338244.2021.1893628. [DOI] [PubMed] [Google Scholar]
- 129.Babaoglu UT, Oymak Yalcin S, Calis AG, Ozgunaltay Ertugrul G, Erturk A. Effects of different occupational exposure factors on the respiratory system of farmers: the case of Central Anatolia. J Public Health. 2022;30(9):2123–31. 10.1007/s10389-021-01554-6. [Google Scholar]
- 130.Shao M, Yang S, Zheng A, Wu Z, Chen M, Yao R, Shi Y, Chen G. Pathophysiological changes in Rhesus monkeys with Paraquat-Induced Pulmonary Fibrosis. Lung. 2022;200(5):549–60. 10.1007/s00408-022-00572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li L, Lv S, Li X, Liu J. Wnt-Induced secreted Proteins-1 play an important role in Paraquat-Induced pulmonary fibrosis. BMC Pharmacol Toxicol. 2022;23(1):21. 10.1186/s40360-022-00560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan B, Chen F, Xu L, Xing J, Wang X. HMGB1-TLR4-IL23-IL17A axis promotes Paraquat-Induced acute lung injury by mediating neutrophil infiltration in mice. Sci Rep. 2017;7(1):597. 10.1038/s41598-017-00721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang X. Molecular mechanism of Paraquat-Induced ferroptosis leading to pulmonary fibrosis mediated by Keap1/Nrf2 signaling pathway. Mol Biol Rep. 2023;50(11):9249–61. 10.1007/s11033-023-08756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cai Q, Jin Y, Jia Z. Paraquat induces lung Injury via miR-199-Mediated SET in a mouse model. Front Pharmacol. 2022;13:856441. 10.3389/fphar.2022.856441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fan L, Xu J, Lv T, Lu M. Asthma attacks: patients who survived paraquat poisoning. J Toxicol Sci. 2022;47(4):147–9. 10.2131/jts.47.147. [DOI] [PubMed] [Google Scholar]
- 136.D’Amico R, Monaco F, Fusco R, Siracusa R, Impellizzeri D, Peritore AF, Crupi R, et al. Atrazine inhalation worsen pulmonary fibrosis regulating the nuclear factor-erythroid 2-Related factor (Nrf2) pathways inducing brain comorbidities. Cell Physiol Biochem. 2021;55(6):704–25. 10.33594/000000471. [DOI] [PubMed] [Google Scholar]
- 137.D’Amico R, Monaco F, Fusco R, Peritore AF, Genovese T, Impellizzeri D, Crupi R, et al. Exposure to Atrazine induces lung inflammation through Nrf2-HO1 andBeclin 1/LC3 pathways. Cell Physiol Biochem. 2021;55(4):413–27. 10.33594/000000393. [DOI] [PubMed] [Google Scholar]
- 138.Ganguli A, Choudhury D, Chakrabarti G. 2,4-Dichlorophenoxyacetic acid induced toxicity in lung cells by disruption of the tubulin-Microtubule network. Toxicol Res. 2014;3(2):118. 10.1039/c3tx50082a. [Google Scholar]
- 139.Gao L, Yuan H, Xu E. Toxicology of paraquat and pharmacology of the protective effect of 5-Hydroxy-1-Methylhydantoin on lung injury caused by Paraquat based on Metabolomics. Sci Rep. 2020;10(1):1790. 10.1038/s41598-020-58599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meyer A, Sandler DP, Beane Freeman LE, Hofmann JN, Parks CG. Pesticide exposure and risk of Rheumatoid arthritis among licensed male pesticide applicators in the agricultural health study. Environ Health Perspect. 2017;125(7):077010. 10.1289/EHP1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parks CG, Hoppin JA, De Roos AJ, Costenbader KH, Alavanja MC, Sandler DP. Rheumatoid arthritis in Agricultural Health Study spouses: associations with pesticides and other farm exposures. Environ Health Perspect. 2016;124(11):1728–34. 10.1289/EHP129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parks CG, Costenbader KH, Long S, Hofmann JN, Beane FL, Sandler DP. Pesticide use and risk of systemic autoimmune diseases in the Agricultural Health Study. Environ Res. 2022;209(June):112862. 10.1016/j.envres.2022.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoppin JA, Umbach DM, Long S, London SJ, Henneberger PK, Blair A, Alavanja M, Freeman LE, Sandler DP. Pesticides are associated with allergic and non-allergic wheeze among male farmers. Environ Health Perspect. 2017;125(4):535–43. 10.1289/EHP315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao Y, Guo S, Wang Y, Yu S, Wang M, Lu X, Li Y. Lymphocyte and its CD4 + and CD8 + subgroup changes after paraquat poisoning. Hum Exp Toxicol. 2019;38(9):1024–30. 10.1177/0960327119851252. [DOI] [PubMed] [Google Scholar]
- 145.Shao Y, Zhao Y, Zhu T, Zhang F, Chang X, Zhang Y, Zhou Z. Paraquat preferentially induces apoptosis of late stage effector lymphocyte and impairs memory immune response in mice. Int J Environ Res Public Health. 2019;16(11):2060. 10.3390/ijerph16112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yousefi M, Adineh H, Reverter M, Hamidi MK, Vatnikov YA, Kulikov EV, Hoseinifar SH. Protective effects of Black seed (Nigella Sativa) diet supplementation in common carp (Cyprinus Carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish Shellfish Immunol. 2021;109(February):12–9. 10.1016/j.fsi.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 147.Buchenauer L, Junge KM, Haange S-B, Simon JC, Bergen MV, Hoh A-L, Aust G, Zenclussen AC, Gabriele I, Stangl. Glyphosate differentially affects the allergic Immune response across generations in mice. Sci Total Environ. 2022;850(December):157973. 10.1016/j.scitotenv.2022.157973. [DOI] [PubMed] [Google Scholar]
- 148.Chang J, Liang C, Wang W, Yong L, Mao W, Yang H, Jia X, Song Y. Toxic effects of Atrazine on Immune function in BALB/c mice. Environ Sci Pollut Res. 2021;28(28):37978–94. 10.1007/s11356-021-13360-4. [DOI] [PubMed] [Google Scholar]
- 149.Wang K, Cai M, Sun J, Chen H, Lin Z, Wang Z, Niu Q. Atrazine exposure can dysregulate the immune system and increase the susceptibility against pathogens in honeybees in a dose-dependent manner. J Hazard Mater. 2023;452(June):131179. 10.1016/j.jhazmat.2023.131179. [DOI] [PubMed] [Google Scholar]
- 150.FAO. A scheme and training manual on good agricultural practices (GAP) for fruits and vegetables. 2018. https://www.fao.org/3/i6677e/i6677e.pdf.
- 151.SafetyCulture. 2024. What is Good Agricultural Practices (GAP)? https://safetyculture.com/topics/good-agricultural-practices/#good-agricultural-practices-examples.
- 152.Merfield CN. Integrated weed management in organic farming. In: Advances in resting-state functional MRI. Elsevier; 2023:31–109. 10.1016/B978-0-323-99145-2.00004-5.
- 153.Jabran K, Chauhan BS. Overview and significance of non-chemical weed control. In: Non-chemical weed control. Elsevier; 2018:1–8. 10.1016/B978-0-12-809881-3.00001-2.
- 154.Ambrus A, Yang YZ. Global harmonization of maximum residue limits for pesticides. J Agric Food Chem. 2016;64(1):30–5. 10.1021/jf505347z. [DOI] [PubMed] [Google Scholar]
- 155.OECD. Country pesticide authorities. n.d. https://www.oecd.org/chemicalsafety/pesticide-compliance/country-pesticide-authorities.htm.
- 156.Preechajarn S. The status of glyphosate in Thailand. 2020. https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=The%20Status%20of%20Glyphosate%20in%20Thailand_Bangkok_Thailand_05-06-2020.
- 157.Campos EV, Ratko J, Bidyarani N, Takeshita V, Fraceto LF. Nature-based herbicides and Micro-/Nanotechnology fostering sustainable agriculture. ACS Sustain Chem Eng. 2023;11(27):9900–17. 10.1021/acssuschemeng.3c02282. [Google Scholar]
- 158.Radhakrishnan R, Alqarawi AA, Abd_Allah EF. Bioherbicides: current knowledge on weed control mechanism. Ecotoxicol Environ Saf. 2018;158(August):131–8. 10.1016/j.ecoenv.2018.04.018. [DOI] [PubMed]
- 159.Kaab SB, Rebey IB, Hanafi M, Hammi KM, Smaoui A, Fauconnier ML, De Clerck C, Jijakli MH, Ksouri R. Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara Cardunculus. South Afr J Bot. 2020;128(January):67–76. 10.1016/j.sajb.2019.10.018. [Google Scholar]
- 160.Todero I, Confortin TássiaC, Luft L, Brun T, Ugalde GA, Almeida TCD, Arnemann JA, Zabot GL, Mazutti MA. Formulation of a bioherbicide with metabolites from Phoma Sp. Sci Hort. 2018;241(November):285–92. 10.1016/j.scienta.2018.07.009. [Google Scholar]
- 161.Raffa CM. Bioremediation of agricultural soils polluted with pesticides: a review. Bioengineering. 2021;8(7):92. 10.3390/bioengineering8070092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mohanty SS, Jena HM. Degradation kinetics and mechanistic study on herbicide bioremediation using hyper butachlor-tolerant Pseudomonas putida G3. Process Saf Environ Prot. 2019;125(May):172–81. 10.1016/j.psep.2019.03.014. [Google Scholar]
- 163.Zhu J, Zhao Y, Li X, Wu L, Fu L, Yang N, Jun Yin, and, Huang R. Isolation of 2 simazine-degrading bacteria and development of a microbial agent for bioremediation of simazine pollution. An Acad Bras Ciênc. 2021;93(suppl 3):e20210373. 10.1590/0001-3765202120210373. [DOI] [PubMed]
- 164.Brosler P, Girão AV, Silva RF, Tedim J, Oliveira FJ. Electrochemical advanced oxidation processes using diamond technology: a critical review. Environments. 2023;10(2):15. 10.3390/environments10020015. [Google Scholar]
- 165.Brillas E. Recent development of electrochemical advanced oxidation of herbicides. A review on its application to Wastewater Treatment and Soil Remediation. J Clean Prod. 2021;290(March):125841. 10.1016/j.jclepro.2021.125841. [Google Scholar]
- 166.Ponnuchamy M, Kapoor A, Kumar PS, Dai-Viet N, Vo A, Balakrishnan MM, Jacob. Sustainable adsorbents for the removal of pesticides from water: a review. Environ Chem Lett. 2021;19(3):2425–63. 10.1007/s10311-021-01183-1. [Google Scholar]
- 167.Thorat T, Patle BK, Wakchaure M, Parihar L. Advancements in techniques used for identification of Pesticide residue on crops. J Nat Pesticide Res. 2023;4(June):100031. 10.1016/j.napere.2023.100031. [Google Scholar]
- 168.Westwood JH, Charudattan R, Duke SO, Fennimore SA, Marrone P, Slaughter DC, Swanton C. Weed Management in 2050: perspectives on the future of weed science. Weed Sci. 2018;66(3):275–85. 10.1017/wsc.2017.78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.