Abstract
Background
Paris polyphylla var. yunnanensis (PPY) is commonly used in traditional Chinese medicine formulas and folk families. Nearly more than 100 chemical substances with medicinal values have been reported in PPY, among which steroidal saponins are the main active components. Due to its long growth cycle, the resource of PPY has become too scarce, and the current production capacity of PPY is still far from meeting the market demand. Numerous studies have shown that endophytic bacteria not only promote the production of secondary metabolites in the host plant, but some of them are also able to produce the same secondary metabolites as the host. However, little is known about the endophytic bacteria associated with PPY in different geographic conditions and tissues. In order to compare the endophytic bacterial communities associated with PPY in different geographic conditions and plant tissues, the endophytic bacteria from roots, stems, and leaves of PPY collected from five locations were isolated, and the diversity, richness, and homogeneity of bacterial communities were analyzed, and the dominant genera correlation with polyphyllin content was further investigated.
Results
A total of 268 endophytic bacterial strains were isolated and identified from PPY. The experimental results showed that the isolates belonged to 5 phyla, 7 classes, 14 orders and 39 genera of bacteria, of which the dominant order was Bacillariophyta and the dominant genera were Bacillus, Pseudomonas and Agrobacterium. In general, the differences in the distribution pattern and diversity of endophytic bacteria in PPY were characterized by the highest diversity and richness index of endophytic bacterial communities in Er yuan Qisheng (QS) and the highest evenness index in Dali Fengyi (FY). The diversity, richness and evenness of bacterial communities in terms of tissue state showed a hierarchical pattern of root > stem > leaf. The three optimal genera were positively correlated with polyphyllin content.
Conclusion
The distribution pattern and diversity of endophytic bacteria in PPY were influenced by tissue type and habitat. In addition, three endophytic bacteria (Pseudomonas, Bacllius and Agrobacterium) were positively correlated with the content of polyphylin.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-025-03814-x.
Keywords: Paris polyphylla var. yunnanensis, Polyphyllin, Plant endophytes, Diversity, Geographic conditions, Tissues
Introduction
Paris polyphylla Smith var. yunnanensis (Franch.) Hand.-Mazz. (PPY) is a perennial herb belonging to the genus (Paris) of the lily family (Linnaeus), which has a long history of medicinal use in Yunnan, and has been included in the successive editions of Chinese Pharmacopoeia. The dried rhizomes of PPY serve as a potent medicinal agent, renowned for their efficacy in clearing away heat and detoxification, eliminating swelling and relieving pain, cooling the liver and calming nerves. Steroidal saponins of PPY are widely regarded as the main active ingredients [1–3]. PPY is not only a commonly used drug in traditional Chinese medicine formulas and folk families, but also the main raw materials utilized in over forty types of proprietary Chinese medicines, such as Yunnan Baiyao series, Gongxuining capsule, JiDeSheng snake tablet, and LouLian capsule [4, 5]. Modern studies have also found that PPY possesses multiple pharmacological properties, including anti-tumor, immunomodulatory, anti-virus eliminate inflammation, hemostatic, and vascular regulation effects [6–10].
The utilization of PPY is in serious resource crisis due to its high demand for medicinal herbs, the long cycle of regeneration, and the indiscriminate exploitation for more than 30 years. Artificial cultivation is one of the most effective ways to solve the problem of endangered medicinal plant resources, but the quality of PPY herbs from different origins varies greatly [11, 12]. At present, a large number of studies on the quality of PPY have been conducted domestically and abroad, yet the outcomes of these investigations are still in a nascent phase, lacking definitive conclusions. Therefore, it is imperative to study the factors influencing PPY quality.
Endophytes are microorganisms that reside in the tissues or cellular interstitial spaces of healthy plants during some or all stages of their life cycle and do not cause any visible plant diseases [13]. Recent studies have demonstrated that endophytes in medicinal plants are closely related to the quality of herbs [14–16]. In addition, the type of plant tissue and geographical location significantly impact the distribution patterns and diversity of endophytes [17–19]. In this work, a microbial pure culture method based on 16 S rDNA sequencing technology was employed to isolate and characterize endophytic bacteria of PPY from different origins. Meanwhile, the contents of saponins in PPY from different origins were determined by high performance liquid chromatography (HPLC). The distribution and diversity of endophytic bacteria across different geographical locations and PPY tissues were explored, and the correlation between endophytic bacterial diversity and steroidal saponin content was also elucidated.
Materials and methods
Reagents and herbal medicine samples
Reference standards (≥ 98%) including Polyphyllin I (PPI), Polyphyllin II (PPII), Polyphyllin VI (PPVI), Polyphyllin VII (PPVII) were purchased from the National Institutes for Good and Drug Control (Beijing, China), Polyphyllin H (PPH), Polyphyllin VII (PPVII) and Polyphyllin V (PPV) were purchased from Shanghai Jinsui Bio-Technology Co., ltd. (Shanghai, China). Methanol and acetonitrile (HPLC grade) were purchased from Tedia (Ohio, USA). Deionized water was prepared by a Mili-Q system (Millipore, Milford, MA, USA). The other reagents obtained were analytical grade. The plant samples of PPY were collected from five different localities in Yunnan, namely Yunlong Guanping (GP), Jianchuan Yangcen (YC), Eryuan Niujie (NJ), Eryuan Qisheng (QS) and Dali Fengyi (FY). The information of the samples is shown in Table S1.
High-performance liquid chromatography
0.5 g dry rhizome powder of PPY was weighed accurately, extracted with 4 mL of ethanol (75%, v/v), and treated with ultrasonication for 60 min. The ethanol mixture solution was filtered, and the residue was extracted again by the same procedure. The filtrate was diluted to 10 mL with ethanol. The sample was filtered by a 0.22 μm microporous membrane. Standard stock solutions were further prepared with methanol at different concentrations as follows: 0.236 mg/mL for PPVII, 0.238 mg/mL for PPH, 0.240 mg/mL for PPVI, 0.650 mg/mL for PPII, 0.736 mg/mL for PPVII, 0.660 mg/mL for PPI, and 0.248 mg/mL for PPV. All of these solutions were stored at 4 °C prior to use.
An Agilent ZORBAX Eclipse XDB-C18 column (4.6 mm × 150 mm, 5 μm), thermostat-controlled at 30 °C, was used for the chromatographic separations, and the gradient elution of wat (solvent A) and acetonitrile (solvent B) at a flow rate of 1 mL/min was employed as follows: 0 ~ 39 min (20% ~ 44% B), 39 ~ 52 min (44% ~ 60% B), 52 ~ 60 min (60% ~ 20% B). The injection volume was 10 µL, and the detection wavelength was set at 203 nm for steroidal saponins.
Method validation
Standard stock solutions were diluted to appropriate concentrations with ethanol for the construction of calibration curves. Six standard solutions were injected into the chromatographic system at 1 µL, 2 µL, 4 µL, 6 µL, 8 µL and 12 µL, respectively. Meanwhile, the calibration curves of PPVII, PPH, PPVI, PPII, PPVII, PPI and PPV were constructed by plotting the peak area versus the content (µg). The limit of detection (LOD) and limit of quantification (LOQ) were also examined in terms of signal-to-noise ratios of 3 and 10, respectively. The precision of the method was evaluated by analyzing the standard solutions. The experiment was repeated six times on the same day. The relative standard deviation (RSD) for the peak area of each standard was calculated, separately. To investigate the stability of the sample solutions, a solution prepared from PPY rhizome was stored at room temperature and analyzed for 0, 2, 4, 6, 8, 10, 12 and 24 h. The recovery test was performed by spiking known concentration levels of the mixture standard solution into known amounts of samples.
Endophyte isolation
The sediment and other impurities on the surface of PPY samples were washed and rinsed three times under running water. The surface of PPY sample was sterilized with alcohol (75%, v/v) and HgCl2 (0.2%, wt.) solution, immersed for 0.5 ~ 1.0 min and 15 ~ 20 min, respectively, and then rinsed with sterile water until the sterile water rinsed was free of microorganism growth [20].
The sterilized PPY samples were chopped, and 5.0 g PPY was grinded until homogeneous, and 5.0 mL sterile water was added for mixing to obtain the PPY suspension. The concentration of the sample suspension was diluted to 10− 1, 10− 2 and 10− 3 g/mL by the multiplicative dilution method. 200 µL diluted suspension was aspirated, spread evenly on the R2A agar medium, and then incubated at 28 ± 2 °C for 2 ~ 4 days in bacteriological incubator. The bacterial colonies (pure colonies) were picked and streaked on the fresh nutrient agar for the selection of clone. The isolated strains were stored in cryopreservation tube containing glycerol at -80 °C.
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted using a Rapid bacterial Genomic DNA Isolation Kit (Sangon Biotech Co., Ltd., Shanghai, China). The 16 S rRNA gene was amplified from the isolates using 27 F and 1492R primer sets [21]. The PCR amplification was performed in a 25 µL reaction system containing 2 µL DNA template, 1 µL of each primer, 8.5 µL ddH2O, and 12.5 µL 2 × Taq PCR Master Mix (Sangon, Biotech Company, Limited, Shanghai, China). The PCR products were verified by agarose gel electrophoresis and purified with a DiaSpin PCR Product Purification Kit (Sangon Biotech Company, Limited, Shanghai, China). The purified PCR products were sequenced in the forward and reverse directions using the same PCR primer pairs at a commercial sequencing provider (Sangon Biotech Company, Limited, Shanghai, China).
Data analysis
Relative frequency (RF) refers to the ratio of the number of endophytic bacterium strains to the total number of isolated strains. Shannon-Wiener diversity index (H’) and Simpson diversity index (D) were used to analyze the biodiversity of endophytic bacteria. Pielou homogeneity index (J) was used to study the homogeneity of the species distribution in different parts of the same species, and the species richness was assessed by calculating Margalet index (R). Determination of dominant colonies: species were considered dominant if Pi > 1/S, where Pi was the relative abundance of species (i) and S was the total number of species present in the community. Pearson’s correlation analysis was performed using IBM SPSS Statistics 25.0 to investigate the correlation between polyphyllin content of PPY and the dominant taxa (genus level) among the five provenances.
Results
Validation of method
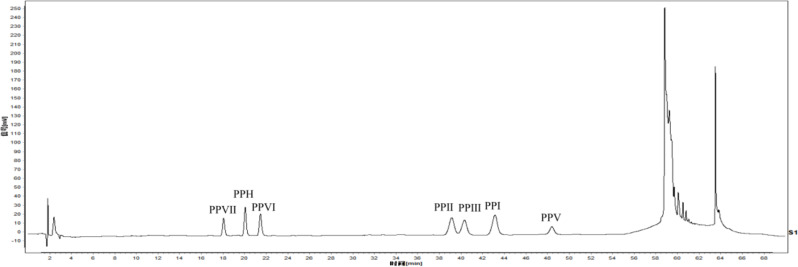
Figure 1 displays the chromatogram of seven standards obtained through HPLC. The regression equation and linear ranges of seven analytes were determined using the developed HPLC method. The results showed that the test solution was well separated from the impurities and without interference. The correlation coefficient values (r2) indicated appropriate correlations between the investigated compound concentration and their peak area within the test ranges. As presented in Table S2, all R2 values obtained using linear regression analysis were greater than 0.99. The average peak areas of PPVII, PPH, PPVI, PPII, PPVII, PPI, and PPV were 141.85, 179.63, 107.75, 321.20, 263.43, 257.55, 229.15, and the relative standard deviations were 0.66%, 0.53%, 0.98%, 0.86%, 0.85%, 0.65%, and 0.70%, respectively.
Fig. 1.
The HPLC reference substances of PPY
The relative standard deviation (RSD) of precision for seven compounds was less than 2%. The RSD values for repeatability and stability of the compounds were all less than 2%, suggesting the high repeatability and stability of the method used. The average recovery was in the range of 98.34 ~ 99.34% with RSD varying from 0.08 to 0.25% (Table S3), indicating that the method used was accurate and reproducible for quantifying the seven steroidal saponins present in PPY samples.
Structure and distribution of endophytic bacterial flora in PPY
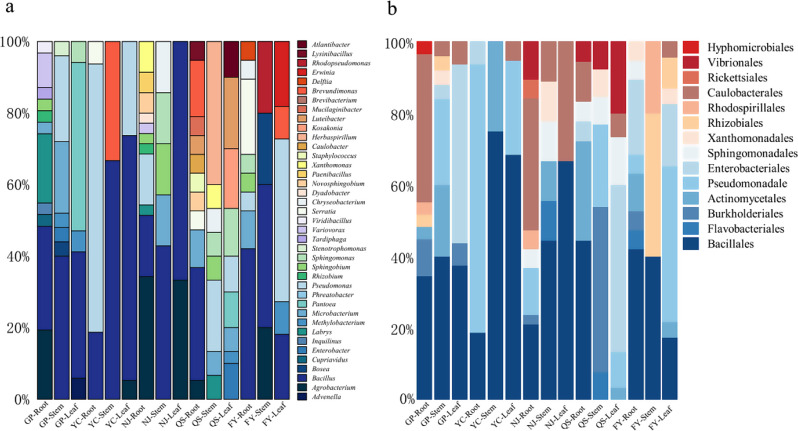
A total of 268 culturable endophytic bacteria were successfully isolated from PPY tissues sourced from five distinct origins. Notably, all the bacteria isolated remained uncontaminated under the specified culture conditions. The sequences generated were checked and assembled using SeqMan Pro v.11 (DNAStar Lasergene, Madison, WI, USA), and then subjected to BLASTn searches in the NCBI (National Center for Biotechnology Information) GenBank nucleotide database. The identification and classification of the thirty-nine taxa is presented in Table S4. Among the isolated bacteria, 165 specimens (comprising 61.57% of the total) were classified as Proteobacteria, while 80 specimens (accounting for 29.85% of the total) belonged to the Firmicutes phylum. Most of them were assigned to 14 orders within 7 classes (Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Bacilli, Actinobacteria, Flavobacteria, Acidobacteria). The main order is Bacillales, and the main genera are Bacillus and Pseudomonas (Fig. 2).
Fig. 2.
Relative frequency (RF) of bacterial groups (a genera, b orders) across PPY host tissues and different sites (GP Guanping, YC, Yangcen, NJ, Niujie, QS, Qisheng, FY, Fengyi)
Differences in endophytic bacteria between regions and PPY tissues
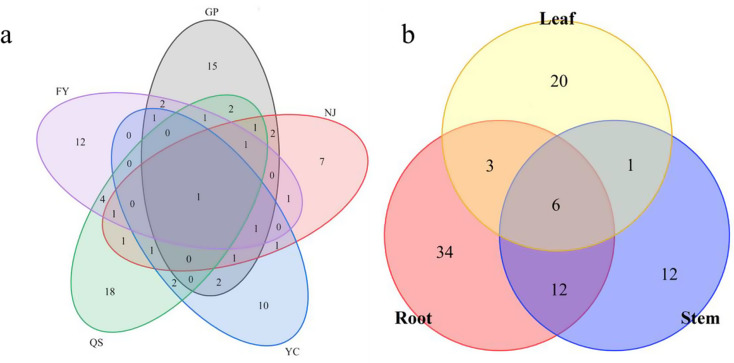
The highest number of endophytic bacterial isolates were collected from GP (73), followed by QS (62), NJ (47), FY (47), and YC (39). The differences in the distribution of endophytic bacteria were observed across the above-mentioned five regions. At the genus level, Bacillus emerged as the dominant genus in GP, YC, and FY, accounting for 34.25%, 48.71%, and 29.78% of the RF, respectively. In contrast, Agrobacterium was the predominant genus in NJ, comprising 25.53% of the RF, while Luteibacter was the dominant genus in QS, with an RF of 11.29%. The endophytic bacteria exhibited region-specificity at the species level, where Pseudomonas poae was prevalent in both roots and leaves, whereas Sphingomonas taxi was commonly associated with stems and leaves (Fig. 3). As presented in Table 1, the differences in endophytic bacterial diversity (H’ and D), evenness (J) and richness (R) were observed across different regions. The bacterial species diversity at YC exhibited the lowest values (H’ = 2.5156, D = 0.9123), and the bacterial species evenness at NJ was the lowest (J = 0.8841). Additionally, the lowest bacterial richness (R = 4.3673) was observed at YC.
Fig. 3.
Wayne diagram of endophytic bacterial strain distribution in PPY(a sites, b tissues)
Table 1.
Species richness, evenness and diversity indices of endophytic bacteria isolated from PPY at different sites
| sites | Shannon’s diversity index (H′) | Simpson’s diversity index (D) | Pielou evenness index (J) | Margalet richness index (R) |
|---|---|---|---|---|
| Guanping | 3.1179 | 0.9581 | 0.8917 | 7.4584 |
| Yangcen | 2.5156 | 0.9123 | 0.8879 | 4.3673 |
| Niujie | 2.7326 | 0.9184 | 0.8840 | 5.4543 |
| Qisheng | 3.2799 | 0.9619 | 0.9310 | 7.9959 |
| Fengyi | 2.9697 | 0.9501 | 0.9471 | 5.7141 |
The roots of PPY exhibited the highest number of endophytic bacterial isolates, with a total of 73 distinct strains identified, whereas the stems displayed the lowest number of such isolates, comprising merely 55 strains. Besides, the diversity (H’ and D) and richness (R) of endophytic bacteria showed significant differences in different tissues (Table 2). The roots possessed the greatest bacterial species diversity (H’ = 3.8631), whereas the leaves exhibited the lowest (H’ = 2.8445). Conversely, according to the Simpson’s index (D), the stems exhibited the highest bacterial species diversity (D = 0.9691), and the roots displayed the lowest diversity (D = 0.9492). The assessment of bacterial species richness (R) revealed that the roots possessed the highest level (R = 11.5562), whereas the leaves exhibited the lowest richness (R = 6.1206).
Table 2.
Indices of endophytic bacteria species richness, evenness, and diversity isolated from different PPY tissues
| sites | Shannon’s diversity index (H′) | Simpson’s diversity index (D) | Pielou evenness index (J) | Margalet richness index (R) |
|---|---|---|---|---|
| Root | 3.8631 | 0.9492 | 0.9640 | 11.5562 |
| Stem | 3.3256 | 0.9691 | 0.9431 | 8.2349 |
| Leaf | 2.8445 | 0.9555 | 0.8447 | 6.1206 |
Correlation between endophytic bacterial communities and steroidal saponin content
The total contents of PPI, PPII and PPVII in PPY samples sourced from five distinct origins varied between 1.0917 and 1.8656%, all of which conformed to the specifications outlined in the 2020 edition of the Chinese Pharmacopoeia. Notably, the concentrations of PPI, PPII and PPVII in the YC and FY samples surpassed the stipulated standards by a margin of 2.5 to 3.1 times. The content of PPIII in the PPY derived from Guanping exhibited a significant increase (p < 0.05) or a highly significant elevation (p < 0.01) compared with those sourced from other origins. Besides, it was the sole origin in which PPV was detectable, with a concentration of 0.1070% (Table S5). The analysis of Pearson’s correlation coefficient was conducted using SPSS software to assess the relationship between the dominant flora and steroidal saponins present in PPY samples originating from five distinct locations. The results are shown in Table 3. A highly significant positive correlation was observed between Bacllius and the total content of PPI, II and VII (p < 0.01). The Pseudomonas displayed a strong positive correlation with the total content of PPI, II, and VII (p < 0.05). Additionally, the Agrobacterium had high-positive correlation with the accumulation of PPH content (p < 0.05).
Table 3.
Correlation analysis between endophytic bacteria and polyphyllin contents
| Genus | PPI | PPII | PPIII | PPVI | PPVII | PPH | PPI+II+VII |
|---|---|---|---|---|---|---|---|
| Bacllius | 0.798 | 0.739 | 0.388 | -0.198 | 0.275 | -0.582 | 0.974** |
| Agrobacterium | -0.643 | -0.799 | -0.651 | 0.680 | -0.064 | 0.885* | -0.851 |
| Pseudomonas | 0.703 | 0.705 | 0.582 | 0.093 | 0.433 | -0.380 | 0.935* |
Note *means p < 0.05, ** means p < 0.01
Discussion
In this study, the diversity of endophytic bacteria in three PPY tissues from five different origins was documented. A total of 268 bacterial isolates were obtained and identified. The experimental results indicated that the isolates belonged to 5 bacterial phyla, 7 classes, 14 orders, and 39 genera. The distribution of endophytic bacteria varied across different locations, which may be attributed to the influence of both biotic and abiotic factors on endophytic bacterial communities [22–26]. These factors ultimately determine their species composition, community structure, diversity, and functionality [27]. It has been found that environmental factors not only impact the distribution of medicinal plants, but also dictate the species composition of bacterial and endophytic fungi [28, 29], and the composition of plant microbial communities is associated with the host species, living environment, plant genetics, and various tissue states [30–33]. The experimental results were consistent with the previous reports on PPY and various host plant species, the distribution of endophytic bacteria in the three tissues varied considerably [34–38]. It is noteworthy that we adopted a culture-dependent isolation method. Due to the inability of laboratory conditions to fully simulate natural environments, some microorganisms exhibit “unculturability”, which may lead to the omission of endophytic bacteria in the analysis, thereby causing deviations in the results of geographical and organizational analysis.
PPY is used in different pharmaceutical systems for the treatment of several diseases. According to reports, nearly 100 plant chemicals with medicinal value have been discovered in PPY, among which steroidal saponin and polyphyllin are the key compounds. However, the current production of resorcylic acid saponins is far from meeting market demand. Many studies have shown that endophytic bacteria can increase the production of polyphyllin by regulating the expression of relevant genes in PPY [39, 40]. The experimental results revealed that three endophytic bacterial genera were associated with the polyphyllin content in PPY. Specifically, Pseudomonas and Bacllius were significantly and positively correlated with the total content of PP I, II and VII, respectively, while Agrobacterium showed a strong positive correlation with the content of PPH. Research has found that inoculation with three potassium-solubilizing bacteria (KSB) (Bacillus thuringiensis, B. polymyxa, and Paenibacillus amylolyticus) can increase the content of Pseudo-protodiosgenin and diosgenin H [41]. Bacillus cereus LgD2 may effectively promote the accumulation of polyphyllin by regulating key downstream genes in biosynthetic pathways [40]. However, considering that endophytes enhance the secondary metabolites of medicinal plants by a variety of factors, they not only can directly promote the production of metabolites in the host plant but also synthesize the same secondary metabolites as the host, and secrete biologically active secondary metabolites that can improve the tolerance of the host plant to biotic stresses and regulate the expression of related genes in the host [42, 43]. Therefore, comprehensive and in-depth studies are required to elucidate the mechanistic relationships between these endophytes and polyphyllin.
Conclusions
The distribution pattern and diversity of endophytic bacteria in PPY were influenced by tissue type and habitat. In addition, three endophytic bacteria (Pseudomonas, Bacllius and Agrobacterium) were positively correlated with the content of polyphylin, and could be used as potential candidates for producing bioactive compounds. This work will facilitate the development of an alternative method for the production of secondary metabolites from endophytes. It is imperative to pay more attention to the interaction between medicinal plants and microorganisms, and enhance research efforts to elucidate the interaction mechanisms, so as to make better use of endophytic resources, and provide theoretical guidance for high-quality and high-yield medicinal plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by “the Expert Workstation of Jiang Yong Yunnan Province (202305AF150048), the Central Government Guidance Fund for Local Scientific and Technological Development(202407AB110009)and 2024 Expert grassroots scientific research workstation in Yunnan Province.
Author contributions
Qing Shu: guided the completion of this experiment, completed the first draft paper, and made revisions. Anzhong Peng and Haifeng Li: guided the experimental methods and thesis writing. Liping Ruan, Jing Wang and Siman Gu: performed experiments, recorded data, and data analysis. Lin Jin and Yuying Wu: data analysis. All authors read and approved the final manuscript.
Funding
This work was supported by “the Expert Workstation of Jiang Yong Yunnan Province (202305AF150048), the Central Government Guidance Fund for Local Scientific and Technological Development (202407AB110009) and 2024 Expert grassroots scientific research workstation in Yunnan Province.
Data availability
The sequences generated in this study were deposited in the NCBI database https://www.ncbi.nlm.nih.gov/ under accession numbers PQ607901 to PQ607917, PQ608572 to PQ608596 and PQ608624 to PQ608658.
Declarations
Ethics approval and consent to participate
Not applicable.
Clinical trial number
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Shu and Liping Ruan contributed equally to this work.
Contributor Information
Anzhong Peng, Email: penganzhong@dali.edu.cn.
Haifeng Li, Email: lihaifeng@dali.edu.cn.
References
- 1.Ding YG, Zhao YL, Zhang J, Zuo ZT, Zhang QZ, Wang YZ. The traditional uses, phytochemistry, and pharmacological properties of Paris L. (Liliaceae): a review. J Ethnopharmacol. 2021;278:114293. 10.1016/j.jep.2021.114293. [DOI] [PubMed] [Google Scholar]
- 2.Man S, Zhang X, Xie L, Zhou Y, Wang G, Hao R, Gao W. A new insight into material basis of rhizoma Paridis saponins in alleviating pain. J Ethnopharmacol. 2024;323:117642. 10.1016/j.jep.2023.117642. [DOI] [PubMed] [Google Scholar]
- 3.Qin XJ, Yu MY, Ni W, Yan H, Chen CX, Cheng YC, et al. Steroidal saponins from stems and leaves of Paris polyphylla var. Yunnanensis. Phytochemistry. 2016;121:20–9. 10.1016/j.phytochem.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Lan PX, He P, Yang J, Zhou GH, Chen XJ, Wei TY, et al. High-throughput sequencing reveals the presence of novel and known viruses in diseased Paris yunnanensis. Front Microbiol. 2022;13:1045750. 10.3389/fmicb.2022.1045750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Liu H, Liu XT, Xu DR, Chen XQ, Wang Q. Qualitative and quantitative analysis of steroidal saponins in crude extracts from Paris polyphylla var. Yunnanensis and P. polyphylla var. Chinensis by high performance liquid chromatography coupled with mass spectrometry. J Pharm Biomed Anal. 2010;51(1):114–24. 10.1016/j.jpba.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Cong Y, Liu X, Kang L, Yu Z, Zhao Z, Li J, et al. Pennogenin tetraglycoside stimulates secretion-dependent activation of rat platelets: evidence for critical roles of adenosine diphosphate receptor signal pathways. Thromb Res. 2012;129(5):e209–16. 10.1016/j.thromres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Jing S, Wang Y, Li X, Man S, Gao W. Chemical constituents and antitumor activity from Paris polyphylla Smith var. yunnanensis. Nat Prod Res. 2017;31(6):660–6. 10.1080/14786419.2016.1219861 [DOI] [PubMed]
- 8.Li L, Zhang J, Cheng W, Di F, Wang C, An Q. Saponins of Paris polyphylla for the improvement of Acne: anti-inflammatory, antibacterial, antioxidant and Immunomodulatory effects. Molecules. 2024;29(8). 10.3390/molecules29081793. [DOI] [PMC free article] [PubMed]
- 9.Liu Z, Li N, Gao W, Man S, Yin S, Liu C. Comparative study on hemostatic, cytotoxic and hemolytic activities of different species of Paris L. J Ethnopharmacol. 2012;142(3):789–94. 10.1016/j.jep.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Liu Y, Wang Y, Gao W. Effect of Rhizoma Paridis saponin on the pain behavior in a mouse model of cancer pain. RSC Adv. 2018;8(31):17060–72. 10.1039/c8ra00797g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Jin H, Zhang J, Zhang J, Wang Y. Quantitative evaluation and discrimination of wild Paris polyphylla var. Yunnanensis (Franch.) Hand.-Mazz from three regions of Yunnan Province using UHPLC-UV-MS and UV spectroscopy couple with partial least squares discriminant analysis. J Nat Med. 2017;71(1):148–57. 10.1007/s11418-016-1044-7. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Zhang J, Jin H, Zhang J, Wang Y. Quantitative analysis in combination with Fingerprint Technology and Chemometric Analysis Applied for evaluating six species of wild Paris using UHPLC-UV-MS. J Anal Methods Chem. 2016;2016:3182796. 10.1155/2016/3182796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mushtaq S, Shafiq M, Tariq MR, Sami A, Nawaz-Ul-Rehman MS, Bhatti MHT, et al. Interaction between bacterial endophytes and host plants. Front Plant Sci. 2022;13:1092105. 10.3389/fpls.2022.1092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou QZ, Chen DW, Wang YP, Ehmet N, Ma J, Sun K. Analysis of endophyte diversity of two Gentiana plants species and the association with secondary metabolite. BMC Microbiol. 2022;22(1):90. 10.1186/s12866-022-02510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju M, Zhang Q, Wang R, Yan S, Li Z, Li P, Gu P. Correlation in endophytic fungi community diversity and bioactive compounds of Sophora alopecuroides. Front Microbiol. 2022;13:955647. 10.3389/fmicb.2022.955647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang B, Yin D, Zhai Y, He A, Qiu L, Liu Q, et al. Diversity of endophytic fungal community in Huperzia serrata from different ecological areas and their correlation with Hup A content. BMC Microbiol. 2022;22(1):191. 10.1186/s12866-022-02605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui XX, Wang L, Fang HY, Zheng YG, Su CY. The cultivable endophytic fungal community of Scutellaria baicalensis: diversity and relevance to flavonoid production by the host. Plant Signal Behav. 2022;17(1):2068834. 10.1080/15592324.2022.2068834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahromi MS, Azizi A, Soltani J. Diversity and Spatiotemporal Distribution of Fungal Endophytes Associated with Salvia Multicaulis. Curr Microbiol. 2021;78(4):1432–47. 10.1007/s00284-021-02430-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhao R, Zheng S, Hu Y, Li H, Chen Y, Chun Z. Endophytic bacterial diversity of the medicinal orchid Dendrobium Nobile. South Afr J Bot. 2023a;158:90–7. 10.1016/j.sajb.2023.04.050. [Google Scholar]
- 20.Lu J, Wang J, Zhang J, Zhu Y, Qin L, Zhu B. Diversity of Culturable Endophytic Fungi in Crocus sativus and their correlation with Crocin Content. Curr Microbiol. 2023;80(2):73. 10.1007/s00284-023-03177-4. [DOI] [PubMed] [Google Scholar]
- 21.Dorsch M, Lane D, Stackebrandt E. Towards a phylogeny of the genus Vibrio based on 16S rRNA sequences. Int J Syst Bacteriol. 1992;42(1):58–63. 10.1099/00207713-42-1-58. [DOI] [PubMed] [Google Scholar]
- 22.Louisson Z, Gutierrez-Gines MJ, Taylor M, Buckley HL, Hermans SM, Lear G. Soil conditions are a more important determinant of microbial community composition and functional potential than neighboring plant diversity. Iscience. 2024;27(6). 10.1016/j.isci.2024.110056. [DOI] [PMC free article] [PubMed]
- 23.Williams G, Miller R, Deng SP. Dynamic relationships between microbial community, enzyme activity, and soil properties across global ecosystems. Appl Soil Ecol. 2025;206. 10.1016/j.apsoil.2024.105843.
- 24.Cuartero J, Querejeta JI, Prieto I, Frey B, Alguacil MM. Warming and rainfall reduction alter soil microbial diversity and co-occurrence networks and enhance pathogenic fungi in dryland soils. Sci Total Environ. 2024;949. 10.1016/j.scitotenv.2024.175006. [DOI] [PubMed]
- 25.Jiang S, Xue D, Feng W, Wang K, Wang S, Wang T, et al. Long-term organic fertilization alters soil microbial community structure and its influence on faba bean production in a six-crop rotation system. Plant Soil. 2024. 10.1007/s11104-024-07169-6. [Google Scholar]
- 26.Liu B, Yang J, Lu W, Wang H, Song X, Yu S, et al. Altitudinal variation in rhizosphere microbial communities of the endangered plant Lilium tsingtauense and the environmental factors driving this variation. Microbiol Spectr. 2024;12(11). 10.1128/spectrum.00966-24. [DOI] [PMC free article] [PubMed]
- 27.Walitang DI, Kim CG, Kim K, Kang Y, Kim YK, Sa T. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biol. 2018;18(1):51. 10.1186/s12870-018-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Liu L, Lu Y, Chen L, Zhang Z, Zhang H, et al. When microclimates meet soil microbes: temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol Biochem. 2022;166. 10.1016/j.soilbio.2022.108566.
- 29.Zhao Y, Zhou Y, Jia X, Han L, Liu L, Ren K, et al. Soil characteristics and microbial community structure on along elevation gradient in a Pinus armandii forest of the Qinling Mountains, China. For Ecol Manag. 2022;503. 10.1016/j.foreco.2021.119793.
- 30.Christian N, Sullivan C, Visser ND, Clay K. Plant Host and Geographic Location Drive Endophyte Community Composition in the Face of Perturbation. Microb Ecol. 2016;72(3):621–32. 10.1007/s00248-016-0804-y. [DOI] [PubMed] [Google Scholar]
- 31.Kandel SL, Joubert PM, Doty SL. Bacterial endophyte colonization and distribution within plants. Microorganisms. 2017;5(4). 10.3390/microorganisms5040077. [DOI] [PMC free article] [PubMed]
- 32.Liu TH, Zhou Y, Tao WC, Liu Y, Zhang XM, Tian SZ. Bacterial diversity in roots, stems, and Leaves of Chinese Medicinal Plant Paris polyphylla var. Yunnanensis. Pol J Microbiol. 2020;69(1):91–7. 10.33073/pjm-2020-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezki S, Campion C, Simoneau P, Jacques M-A, Shade A, Barret M. Assembly of seed-associated microbial communities within and across successive plant generations. Plant Soil. 2018;422(1–2):67–79. 10.1007/s11104-017-3451-2. [Google Scholar]
- 34.Li Y, Jin J, Li P, Wang Q, Xu L, Wei G, Li Z. Regional variations and plant compartments shape the community structures of the endophytic microbiome and secondary metabolites of Astragalus mongholicus. Ind Crops Prod (Netherlands). 2023;116037. 10.1016/j.indcrop.2022.116037.
- 35.Emiliani G, Mengoni A, Maida I, Perrin E, Chiellini C, Fondi M et al. Linking Bacterial Endophytic Communities to Essential Oils: Clues from Lavandula angustifolia Mill. Evidence-Based Complementary Alt Med. 2014;2014; 10.1155/2014/650905 [DOI] [PMC free article] [PubMed]
- 36.Maggini V, Miceli E, Fagorzi C, Maida I, Fondi M, Perrin E, et al. Antagonism and antibiotic resistance drive a species-specific plant microbiota differentiation in Echinacea spp. FEMS Microbiol Ecol. 2018;94(8). 10.1093/femsec/fiy118. [DOI] [PubMed]
- 37.Maggini V, Mengoni A, Gallo ER, Biffi S, Fani R, Firenzuoli F, Bogani P. Tissue specificity and differential effects on in vitro plant growth of single bacterial endophytes isolated from the roots, leaves and rhizospheric soil of Echinacea purpurea. BMC Plant Biol. 2019;19. 10.1186/s12870-019-1890-z. [DOI] [PMC free article] [PubMed]
- 38.Semenzato G, Del Duca S, Vassallo A, Zaccaroni M, Mucci N, Greco C, et al. Exploring the nexus between the composition of essential oil and the bacterial phytobiome associated with different compartments of the medicinal plants Origanum vulgare ssp. vulgare, O. vulgare ssp. hirtum, and O. heracleoticum. Ind Crops Prod. 2023;191. 10.1016/j.indcrop.2022.115997.
- 39.Chen Y, Yu D, Huo J, Huang N, Zhang M, Du X. Studies on biotransformation mechanism of Fusarium sp. C39 to enhance saponin content of Paridis Rhizoma. Front Microbiol. 2022;13:992318. 10.3389/fmicb.2022.992318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Chang S, Yang Y, Xi C, Dong Y, Liu L, et al. Endophyte-inoculated rhizomes of Paris polyphylla improve polyphyllin biosynthesis and yield: a transcriptomic analysis of the underlying mechanism. Front Microbiol. 2023;14:1261140. 10.3389/fmicb.2023.1261140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao SX, Deng QS, Jiang CY, Wu QS, Xue YB, Li GL et al. Inoculation with Potassium Solubilizing Bacteria and Its Effect on the Medicinal Characteristics of Paris polyphylla var. yunnanensis. Agriculture-Basel. 2023;13(1); 10.3390/agriculture13010021
- 42.Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP. A friendly relationship between Endophytic Fungi and Medicinal plants: a systematic review. Front Microbiol. 2016;7:906. 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venieraki A, Dimou M, Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hellenic Plant Prot J. 2017;10(2):51–66. 10.1515/hppj-2017-0006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences generated in this study were deposited in the NCBI database https://www.ncbi.nlm.nih.gov/ under accession numbers PQ607901 to PQ607917, PQ608572 to PQ608596 and PQ608624 to PQ608658.