Abstract
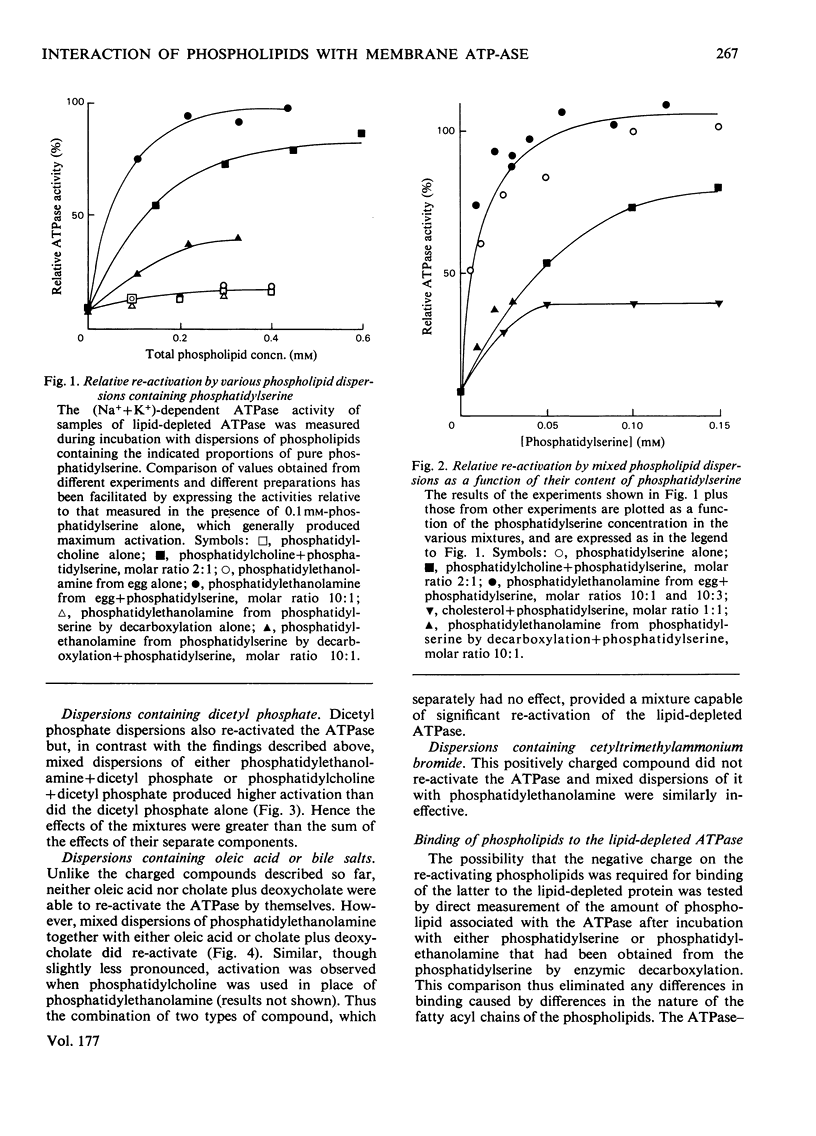
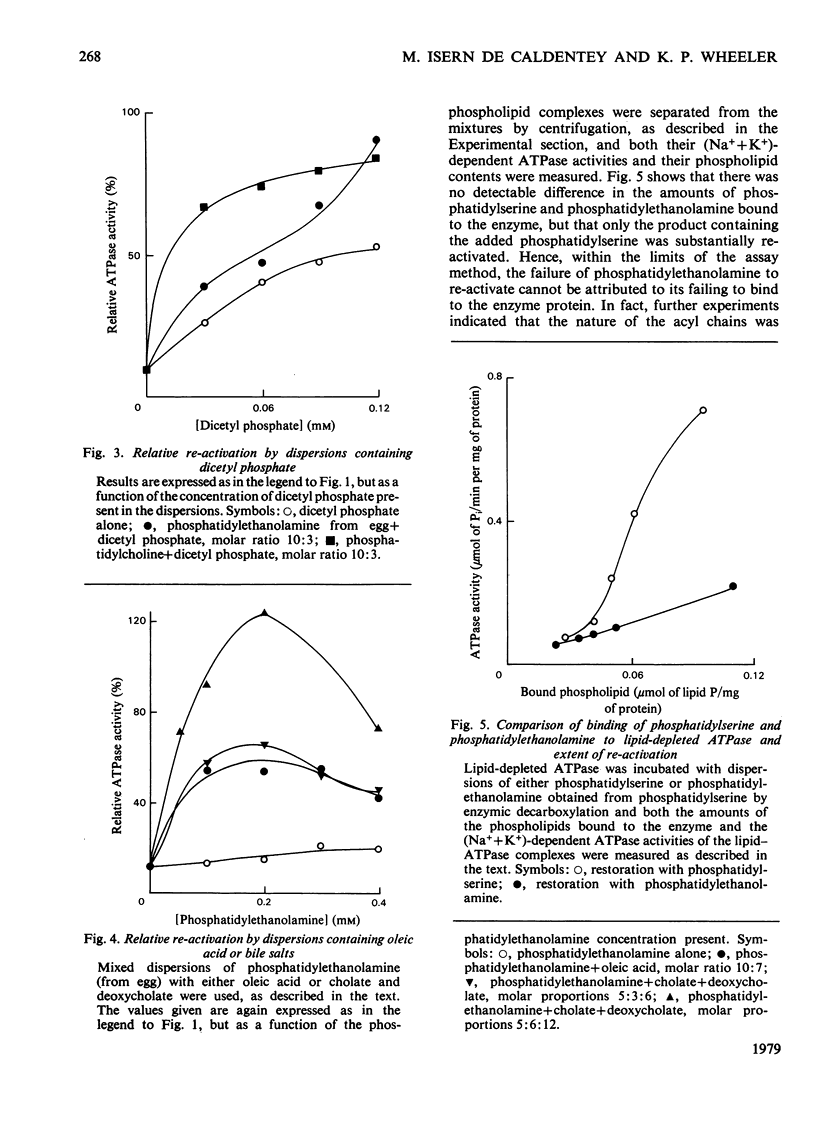
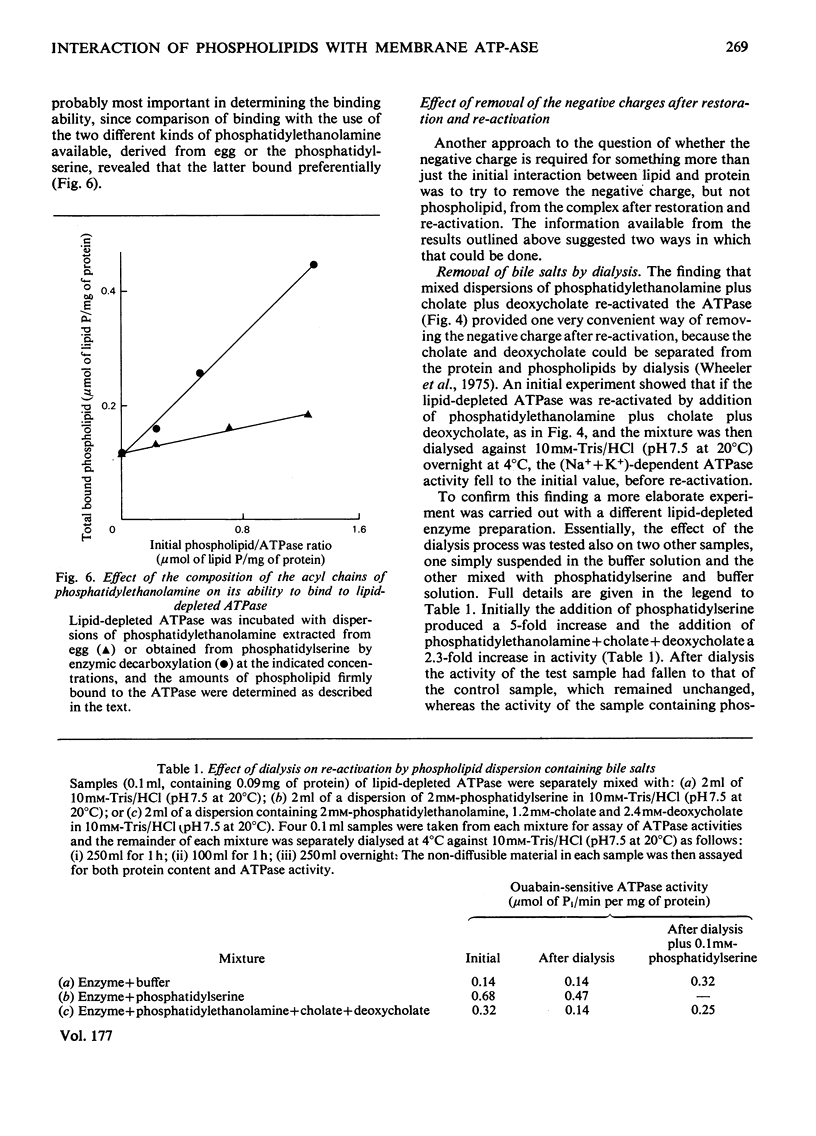
The basis of the requirement for a net negative charge on phospholipid dispersions able to re-activate lipid-depleted (Na++K+)-dependent adenosine triphosphatase was studied. The origin and density of the charge in phospholipid dispersions were varied before interaction with the adenosine triphosphatase protein, and the charge density on restored phospholipid-adenosine triphosphatase complexes was changed after interaction. The results indicated that: (a) re-activation requires a lamellar arrangement of the lipid molecules with sufficient density of negative charge, but not necessarily negatively charged phospholipid molecules; (b) the net charge appears to be necessary for the correct interaction between the enzyme protein and the phospholipids, although the amount of phospholipid that binds to the protein is also a function of the nature of the acyl chains; (c) it is not possible on the basis of these findings and those in the literature to decide unequivocally if the charge is also required for the enzyme reaction itself. The possible relevance of the findings to the situation in vivo is discussed in terms of the charge being concerned only with lipid-protein interaction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Caldentey M. I., Wheeler K. P. Interactions of phospholipids with sodium-plus-potassium ion-dependent adenosine triphosphatase. Biochem Soc Trans. 1977;5(1):107–108. doi: 10.1042/bst0050107. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Isern de Caldentey M., Wheeler K. P. A simple and rapid method for the reversible removal of lipids from a membrane-bound enzyme. Biochem J. 1978 Feb 1;169(2):305–311. doi: 10.1042/bj1690305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilden S., Hokin L. E. Active potassium transport coupled to active sodium transport in vesicles reconstituted from purified sodium and potassium ion-activated adenosine triphosphatase from the rectal gland of Squalus acanthias. J Biol Chem. 1975 Aug 25;250(16):6296–6303. [PubMed] [Google Scholar]
- Hilden S., Hokin L. Coupled Na+ -K+ transport in vesicles containing a purified (NaK)-ATPase and only phosphatidyl choline. Biochem Biophys Res Commun. 1976 Mar 22;69(2):521–527. doi: 10.1016/0006-291x(76)90552-0. [DOI] [PubMed] [Google Scholar]
- Jensen J., Ottolenghi P. Adenosine diphosphate binding to sodium-plus-potassium ion-dependent adenosine triphosphatase. The role of lipid in the nucleotide-potassium ion interplay. Biochem J. 1976 Dec 1;159(3):815–817. doi: 10.1042/bj1590815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Karlsson K. A., Samuelsson B. E., Steen G. O. Structure and function of sphingolipids. 2. Differences in sphingolipid concentration, especially concerning sulfatides, between some regions of bovine kidney. Acta Chem Scand. 1968;22(8):2723–2724. doi: 10.3891/acta.chem.scand.22-2723. [DOI] [PubMed] [Google Scholar]
- Mandersloot J. G., Roelofsen B., de Gier J. Phosphatidylinositol as the endogenous activator of the (Na+ + K+)-ATPase in microsomes of rabbit kidney. Biochim Biophys Acta. 1978 Apr 20;508(3):478–485. doi: 10.1016/0005-2736(78)90093-7. [DOI] [PubMed] [Google Scholar]
- Ottolenghi P. The reversible delipidation of a solubilized sodium-plus-potassium ion-dependent adenosine triphosphatase from the salt gland of the spiny dogfish. Biochem J. 1975 Oct;151(1):61–66. doi: 10.1042/bj1510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P., Dabbeni-Sala F., Pitotti A., Bruni A., Mandersloot J. C. Activation of (Na+ + K+)-dependent ATPase by lipid vesicles of negative phospholipids. Biochim Biophys Acta. 1977 Apr 1;466(1):1–9. doi: 10.1016/0005-2736(77)90203-6. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Goldin S. M. Reconstitution of active ion transport by the sodium and potassium ion-stimulated adenosine triphosphatase from canine brain. J Biol Chem. 1975 May 25;250(10):4022–4024. [PubMed] [Google Scholar]
- Walker J. A., Wheeler K. P. Polar head-group and acyl side-chain requirements for phospholipid-dependent (Na-+ plus K-+)-ATPase. Biochim Biophys Acta. 1975 Jun 11;394(1):135–144. doi: 10.1016/0005-2736(75)90212-6. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Complete control of the lipid environment of membrane-bound proteins: application to a calcium transport system. FEBS Lett. 1974 Apr 15;41(1):122–124. doi: 10.1016/0014-5793(74)80969-5. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reconstitution of a calcium pump using defined membrane components. Proc Natl Acad Sci U S A. 1974 Mar;71(3):622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K. P., Walker J. A., Barker D. M. Lipid requirement of the membrane sodium-plus-potassium ion-dependent adenosine triphosphatase system. Biochem J. 1975 Mar;146(3):713–722. doi: 10.1042/bj1460713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pont J. J., van Prooijen-van Eeden A., Bonting S. L. Role of negatively charged phospholipids in highly purified (Na+ + K+)-ATPase from rabbit kidney outer medulla studies on (Na+ + K+)-activated ATPase, XXXIX. Biochim Biophys Acta. 1978 Apr 20;508(3):464–477. doi: 10.1016/0005-2736(78)90092-5. [DOI] [PubMed] [Google Scholar]


