Abstract
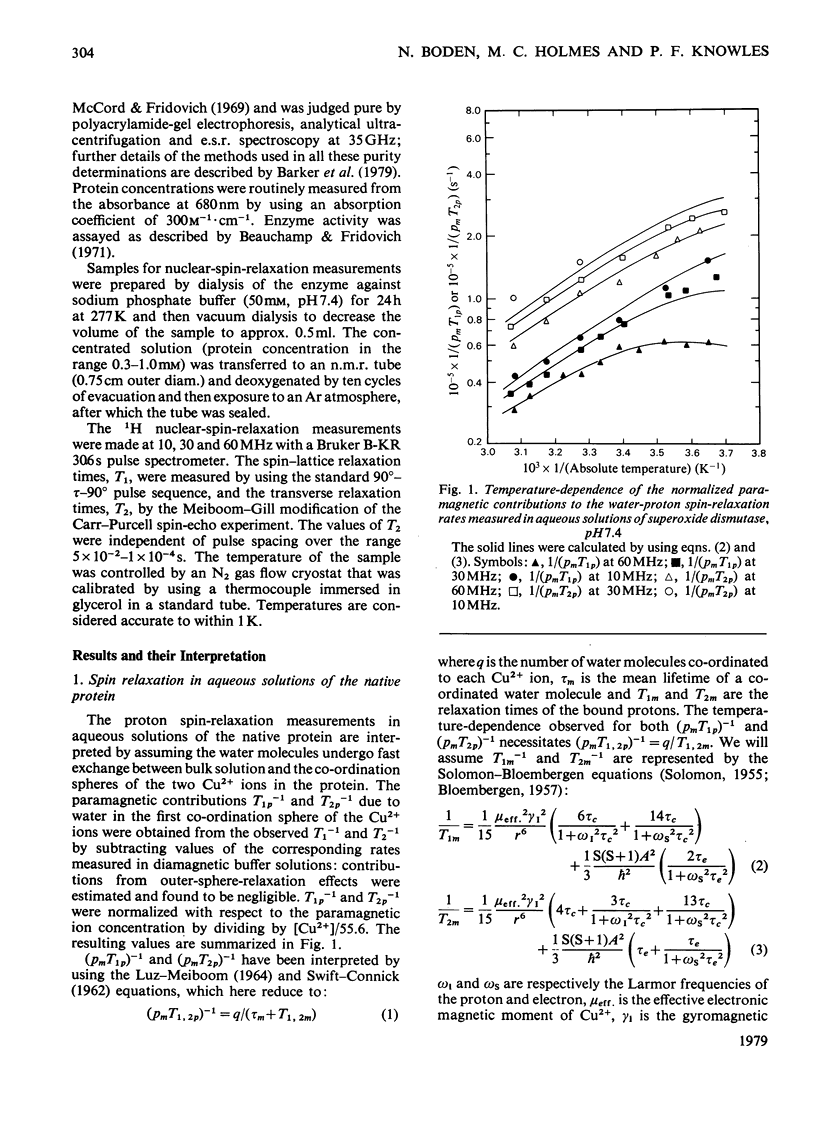
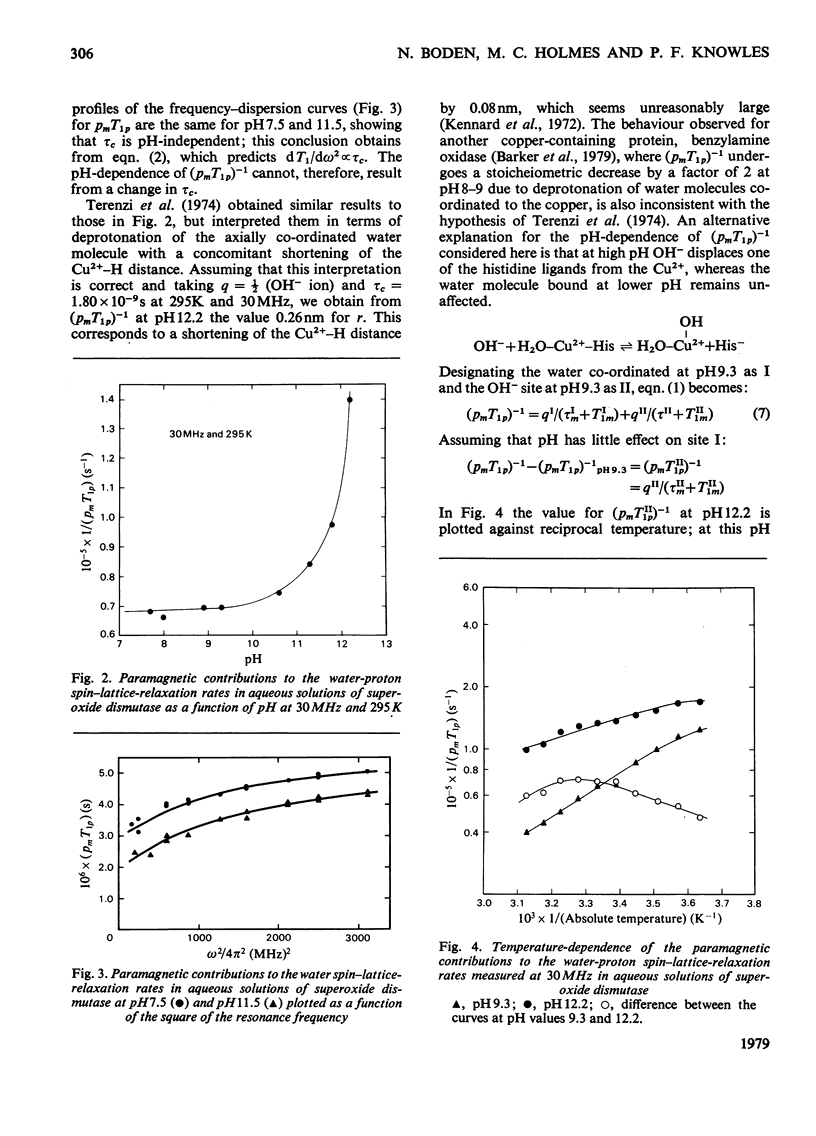
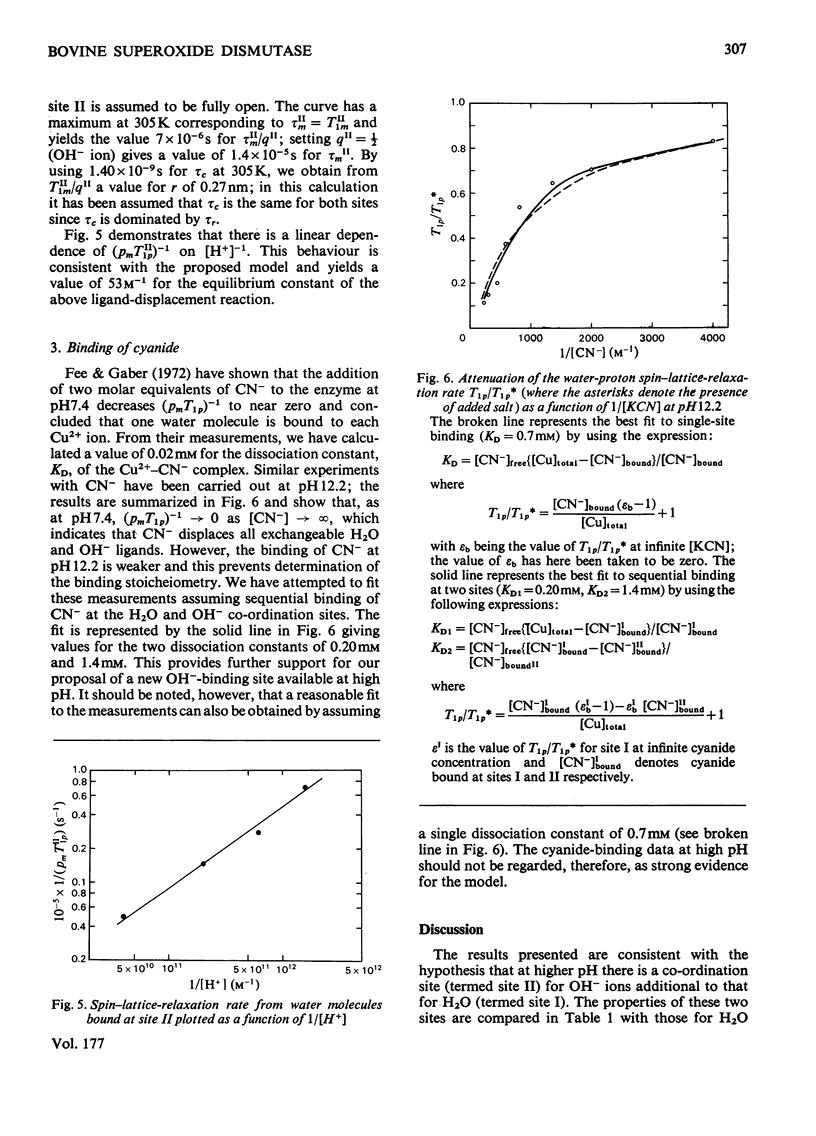
Proton nuclear-relaxation rates have been measured as a function of frequency, temperature, pH and cyanide concentration in aqueous solutions of superoxide dismutase from bovine erythrocytes. The results show that, whereas for pH less than or equal to 9 only one water molecule is bound to each Cu2+ ion, at higher pH a second co-ordination site for OH- becomes available; it is proposed that this involves cleavage of the bond between Cu2+ and the histidine residue that bridges to Zn2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker R., Boden N., Cayley G., Charlton S. C., Henson R., Holmes M. C., Kelly I. D., Knowles P. F. Properties of cupric ions in benzylamine oxidase from pig plasma as studied by magnetic-resonance and kinetic methods. Biochem J. 1979 Jan 1;177(1):289–302. doi: 10.1042/bj1770289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beem K. M., Richardson D. C., Rajagopalan K. V. Metal sites of copper-zinc superoxide dismutase. Biochemistry. 1977 May 3;16(9):1930–1936. doi: 10.1021/bi00628a027. [DOI] [PubMed] [Google Scholar]
- Boden N., Holmes M. C., Knowles P. F. Binding of water to "types I and II" Cu2+ in proteins. Biochem Biophys Res Commun. 1974 Apr 8;57(3):845–848. doi: 10.1016/0006-291x(74)90623-8. [DOI] [PubMed] [Google Scholar]
- Cass A. E., Hill A. O., Smith B. E., Bannister J. V., Bannister W. H. Investigation of the structure of bovine erythrocyte superoxide dismutase by 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1977 Jul 12;16(14):3061–3066. doi: 10.1021/bi00633a003. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Gaber B. P. Anion binding to bovine erythrocyte superoxide dismutase. Evidence for multiple binding sites with qualitatively different properties. J Biol Chem. 1972 Jan 10;247(1):60–65. [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gaber B. P., Brown R. D., Koenig S. H., Fee J. A. Nuclear magnetic relaxation dispersion in protein solutions. V. Bovine erythrocyte superoxide dismutase. Biochim Biophys Acta. 1972 Jun 22;271(1):1–5. doi: 10.1016/0005-2795(72)90126-2. [DOI] [PubMed] [Google Scholar]
- Lippard S. J., Burger A. R., Ugurbil K., Pantoliano M. W., Valentine J. S. Nuclear magnetic resonance and chemical modification studies of bovine erythrocyte superoxide dismutase: evidence for zinc-promoted organization of the active site structure. Biochemistry. 1977 Mar 22;16(6):1136–1141. doi: 10.1021/bi00625a017. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotilio G., Morpurgo L., Giovagnoli C., Calabrese L., Mondovì B. Studies of the metal sites of copper proteins. Symmetry of copper in bovine superoxide dismutase and its functional significance. Biochemistry. 1972 May 23;11(11):2187–2192. doi: 10.1021/bi00761a028. [DOI] [PubMed] [Google Scholar]
- Terenzi M., Rigo A., Franconi C., Calabrese L., Rotilio G., Mondovì B. pH dependece of the nuclear magnetic relaxation rate of solvent water protons in solutions of bovine superoxide dismutase. Biochim Biophys Acta. 1974 Jun 7;351(2):230–236. doi: 10.1016/0005-2795(74)90185-8. [DOI] [PubMed] [Google Scholar]


