Abstract
Background
In the field of global health, meeting the energy requirements of athletes has surfaced as an essential concern. This study seeks to evaluate the effectiveness of royal jelly (RJ) in improving exercise performance in endurance-trained men.
Methods
In this randomized, crossover, double-blind, placebo-controlled study, we will enroll 18 male endurance athletes. Participants will be randomly assigned to one of two conditions: the intervention condition, which will receive royal jelly (RJ) in 500 mg capsules taken orally twice daily for 2 weeks, or the control condition, which will receive a placebo consisting of 500 mg starch capsules taken orally twice daily for the same duration. The study will utilize a 2 × 2 crossover design with a 2-week washout period between treatments. To evaluate aerobic performance, we will determine each participant’s maximal aerobic speed (MAS), followed by a test conducted at 80% of the MAS to measure time to exhaustion. Blood samples (5 cc) will be collected from all participants before and after the treadmill tests to analyze mRNA expressions of Nrf2 and PGC-1α, as well as oxidative stress parameters. Additionally, participants’ dietary intake will be assessed using 3-day food records, and their blood pressure will be monitored before exercise, immediately after, and half an hour post-exercise.
Discussion
This trial aims to evaluate the effectiveness of RJ as a nutraceutical agent for enhancing endurance athletic performance. We anticipate that the results will provide new insights into the clinical and molecular benefits of RJ. Additionally, these findings will offer valuable data to guide the design and execution of future clinical research involving RJ.
Trial registration
This study was registered in the Iranian Registry of Clinical Trials (registration No. IRCT20231209060310N1, date: December 21, 2023).
Keywords: Royal jelly, Athlete, Performance, Oxidative stress, Gene expression
Administrative information
| Title {1} | Effects of Royal Jelly on Oxidative Stress Markers, Athletic Performance, and Gene Expression of Nrf2 and PGC-1α Related to Mitochondrial Biogenesis in Male Endurance Athletes: A Randomized, Crossover, Double-Blind, Placebo-Controlled Trial Protocol |
| Trial registration {2a and 2b}. | Iranian Registry of Clinical Trials (IRCT20231209060310N1( |
| Protocol version {3} | Date: December 21, 2023; Version No: 1 |
| Funding {4} | This study is supported by the Kermanshah University of Medical Sciences, Kermanshah, Iran. |
| Author details {5a} |
Mahsa Miryan1, 2, 3, Vahid Tadibi4, Ehsan Sadeghi5, Farid Najafi6, Amir Saber2, Mohammadreza Abbaspour7, Yahya Pasdar2, 3* 1 Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran 2 Nutritional Sciences Department, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran 3Research Center for Environmental Determinants of Health (RCEDH), Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran 4Exercise Metabolism and Performance Lab (EMPL), Department of Exercise Physiology, Faculty of Sport Sciences, Razi University, University Avenue, Taq-e Bostan, Kermanshah 6714414971, Iran 5Department of Food Science and Technology, School of Nutrition Science and Food Technology, Research Center for Environmental Determinants of Health (RCEDH), Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran 6Research Center for Environmental Determinacies of Health, School of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran 7Targeted Drug Delivery Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran |
| Name and contact information for the trial sponsor {5b} |
Dr. Yahya Pasdar Address: Nutritional Sciences Department, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran. Email: Yahya.pasdar@kums.ac.ir Tel: +98 8337102015 |
| Role of sponsor {5c} | The study sponsor played no role in the study's design, the collection, management, analysis, or interpretation of data, the writing of the report, or the decision to submit the report for publication. Furthermore, they did not have ultimate authority over any of these activities. |
Introduction
Background and rationale {6a}
The nutritional needs of athletes are largely shaped by their training regimens and the specific goals they set to attain optimal performance and maintain overall health [1, 2]. Neglecting these needs can have a profound effect on sports performance. Consequently, coaches are increasingly prioritizing the unique nutritional requirements of athletes, which include adequate intake of energy, protein, fats, minerals, and vitamins [3]. Nutritional ergogenic supplements may enhance performance during physical activities and aid recovery between training sessions or competitions [4]. Even a slight improvement of just 1% in athletic performance can be the difference between securing a medal and finishing out of the top ranks [5].
The heightened demand for oxygen during athletic activities increases the production of reactive oxygen species (ROS), which can result in oxidative stress and subsequent muscle damage. Oxidative stress occurs when the production of ROS exceeds the body’s capacity to neutralize them with antioxidants [6, 7]. These ROS molecules can damage crucial cellular components, including proteins, lipids, and DNA, impairing cellular function and causing tissue injury [8].
Athletes are particularly susceptible to elevated levels of ROS during intense physical exertion, primarily due to an increased metabolic rate and heightened oxygen consumption. This oxidative damage can adversely affect muscle performance, recovery, and endurance [9]. Consequently, antioxidant supplementation may offer significant benefits to athletes by mitigating oxidative stress and enhancing exercise outcomes, including physical performance and stamina [10, 11]. A notable advantage of such supplements is their ability to provide essential antioxidants that help counteract the deleterious effects of ROS generated during vigorous exercise [12]. Antioxidants work by scavenging these harmful molecules and protecting cells from oxidative damage. Common antioxidants utilized by athletes include vitamins C and E, carotenoids, flavonoids, and polyphenols [13]. This strategic supplementation can play an integral role in promoting better performance and recovery in athletic training.
Royal jelly (RJ) is a substance naturally produced by worker bees, renowned for its rich nutrient profile and impressive antioxidant properties. It has been demonstrated to protect cells from oxidative stress and damage caused by ROS, thereby positively influencing athletic performance and recovery [14]. In addition, RJ enhances the body’s external antioxidant defenses through its antioxidant compounds, while also regulating the internal antioxidant system by modulating the expression of antioxidant enzymes and promoting mitochondrial biogenesis [15]. RJ supplementation also has the potential to reduce fatigue and enhance pleasure/arousal by boosting energy metabolism as well as antioxidant defenses, which are crucial for athletic performance [16]. Some research suggests that RJ may lead to modest reductions in body weight (BW) and waist circumference, which could be attributed to its purported anti-inflammatory and metabolic properties [17]. Recent studies have explored the potential effects of RJ supplementation on cardiovascular health. These studies suggest that RJ may have beneficial impacts on inflammation and oxidative stress, which are important factors in cardiovascular health [18]. Additionally, RJ has shown promise in improving vascular endothelial activity [19]. Overall, RJ supplementation appears to offer several potential cardiovascular benefits, although more research is needed to fully understand its effects.
In this study, we aim to assess the effects of RJ supplementation on some oxidative stress markers in the serum of athletes, as well as its influence on aerobic endurance performance, as evaluated through a treadmill test. Additionally, we will examine the expression of genes associated with mitochondrial biogenesis within this population. Our hypothesis posits that RJ supplementation may enhance these oxidative stress markers, potentially mitigating oxidative damage to skeletal muscles caused by exercise. Moreover, we anticipate an improvement in the aerobic capacity of endurance-trained male athletes, alongside a positive modulation of specific genes linked to mitochondrial biogenesis. To test our hypothesis, we have designed a randomized, double-blind, placebo-controlled crossover clinical trial, involving 18 participants in each phase of the study.
Objectives {7}
The objectives of the current clinical trial are to:
Evaluate the effect of RJ supplementation versus placebo on serum oxidative stress parameters, including malondialdehyde (MDA), total antioxidant capacity (TAC), total oxidant status (TOS), and oxidative stress index (OSI), in endurance-trained men
Assess the influence of RJ supplementation compared to placebo on aerobic endurance performance, by measuring time-to-exhaustion (TTE) during exercise at 80% of the maximal aerobic speed (MAS) in endurance-trained men
Investigate the effects of RJ supplementation versus placebo on the expression of genes associated with mitochondrial biogenesis, focusing on Nrf2 and PGC-1α, in endurance-trained men
Analyze the effects of RJ supplementation compared to placebo on anthropometric measurements (BW, body mass index (BMI), waist circumference, and waist-to-hip ratio (WHR)) and body composition in endurance-trained men
Examine the effect of RJ supplementation versus placebo on subjective exercise-related measures, including the Borg rating of perceived exertion (RPE), feelings of pleasure, and arousal, in endurance-trained men
Determine the effects of RJ supplementation compared to placebo on blood pressure levels in endurance-trained men
Explore the influence of RJ supplementation versus placebo on heart rate (HR) responses during exercise in endurance-trained men
Trial design {8}
The current study is designed as a crossover trial with a 1:1 allocation ratio to explore the effect of RJ supplementation on oxidative stress markers, athletic performance, and gene expression of Nrf2 and PGC-1α compared to placebo in male endurance athletes.
Methods: participants, interventions and outcomes
Study setting {9}
The upcoming research will take place in a sports laboratory at the Faculty of Sports Sciences, Razi University in Kermanshah, Kermanshah, Iran.
Eligibility criteria {10}
The inclusion criteria include:
Engaging in endurance exercises at least thrice weekly over the past 6 months
Giving written consent
Aged 18 to 44
Male gender
The exclusion criteria include:
Antioxidant supplements intake (e.g., vitamin E, vitamin C, and selenium), RJ, or anti-inflammatory medications in the past 3 months
Previous acute or chronic respiratory diseases, heart conditions, diabetes mellitus, and metabolic disorders
Consumption of any dietary supplements within the last 3 months
Adherence to particular dietary programs
Presence of musculoskeletal disorders or injuries, history of injuries from training or exercise
Allergic reactions to bee products and RJ
Participants will be recruited from clubs in Kermanshah city, specifically targeting male individuals who have committed to regular endurance training—consisting of a minimum of three sessions per week—for at least 6 months before the study. Eligible athletes will be selected by a sports physiologist (V.T.) and nutritionist (M.M.), and following an initial screening process, they will be randomly assigned to study conditions after providing their voluntary consent.
Who will take informed consent? {26a}
M.M. will provide a detailed explanation of the study to the participants. If they indicate a willingness to participate, she will proceed to obtain informed consent from those interested in joining the trial. The study protocol and written informed consent have been approved by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran (No. IR.KUMS.REC.1402.419). The current trial is in accordance with the Declaration of Helsinki.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable.
Interventions
Explanation for the choice of comparators {6b}
Not applicable.
Intervention description {11a}
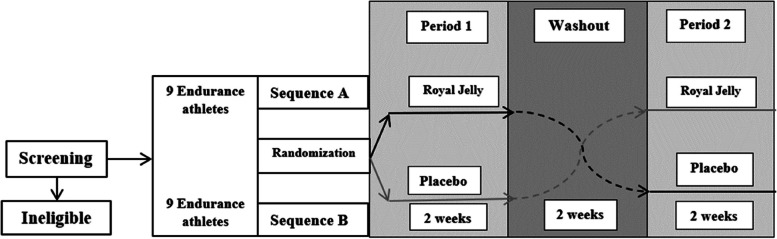
Participants will be randomly assigned to one of two sequences for a 2 × 2 crossover study design. In sequence A, participants will receive the active intervention (RJ) in 500 mg capsules, administered twice daily after breakfast and dinner for 2 weeks. In sequence B, participants will receive a placebo, consisting of 500 mg neutral starch capsules, also taken twice daily for 2 weeks. Throughout the study, participants will be required to record their well-being after each capsule intake to monitor any unexpected effects. After the initial 2-week treatment period, there will be a 2-week washout period before crossing over to the alternate treatment.
Criteria for discontinuing or modifying allocated interventions {11b}
Participants have the freedom to withdraw from the study at any point and for any reason, without facing any repercussions. The research team will carefully monitor adverse events throughout the trial, with oversight from supervisory personnel and regular reporting to the principal investigators. Treatment-related to the study may need to be discontinued for one of the following reasons: (a) if the participant withdraws their consent to continue in the study, or (b) if the investigator determines that treatment should be terminated. An investigator may discontinue a participant’s treatment for specific reasons, such as (a) the emergence of severe complications requiring immediate cessation of treatment, like an acute allergic reaction, or (b) cases of non-compliance with the study protocol. In premature treatment termination, the investigator is responsible for informing the participant of the decision and thoroughly documenting the primary reason for withdrawal in the participant’s file.
Suppose a participant’s actions lead to their withdrawal from the study. In that case, any data collected before the termination may still be utilized, provided that the participant has agreed to this and signed an informed consent form for follow-up. Additionally, any modifications to the assigned interventions will be made in consultation with supervisory personnel if the participant’s condition deteriorates. Furthermore, the study findings will provide a detailed explanation of the reasons for each participant’s withdrawal.
Strategies to improve adherence to interventions {11c}
Participants will receive RJ and placebo capsules at baseline and week 4, along with instructions for their use. They are required to return any remaining capsules at week 2 and the end of the trial. Any side effects experienced will be recorded. A daily supplement consumption checklist will also be provided for participants to bring to their regular visits. Adherence to the intervention will be assessed via phone calls and the count of returned capsules at the trial’s end. Compliance will be calculated using the formula: Compliance rate = (capsules taken/capsules prescribed) × 100, with poor compliance defined as < 80% [20], which will exclude participants from the final analysis. To enhance adherence, we will set up calendar reminders, use social media for daily notifications, and conduct weekly follow-up phone calls.
Relevant concomitant care permitted or prohibited during the trial {11d}
Participants will be instructed not to change their diet, level of physical activity, or use any other RJ-containing products during the study.
Provisions for post-trial care {30}
They are referred to medical centers, where the costs of their treatment are fully covered.
Outcomes {12}
The primary outcome measure is:
The difference in serum levels of MDA between the RJ condition and placebo condition after the 2-week intervention
The secondary outcome measures are:
The difference in serum levels of TAC and TOS between the RJ condition and placebo condition after the 2-week intervention
The difference in OSI between the RJ condition and placebo condition after the 2-week intervention
The difference in aerobic endurance performance through a TTE test conducted at 80% of MAS between the RJ condition and placebo condition after the 2-week intervention
The difference in levels of Nrf2 gene expression between the RJ condition and placebo condition after the 2-week intervention
The difference in levels of PGC-1α gene expression between the RJ condition and placebo condition after the 2-week intervention
The difference in Borg RPE, arousal perceptions, and affective responses between the RJ condition and placebo condition after the 2-week intervention
The difference in blood pressure (pre- and post-exercise) between the RJ condition and placebo condition after the 2-week intervention
The difference in HR (pre- and post-exercise) between the RJ condition and placebo condition after the 2-week intervention
The difference in anthropometrics (BW, BMI, waist circumference, and WHR) between the RJ condition and placebo condition after the 2-week intervention
Participant timeline (1)
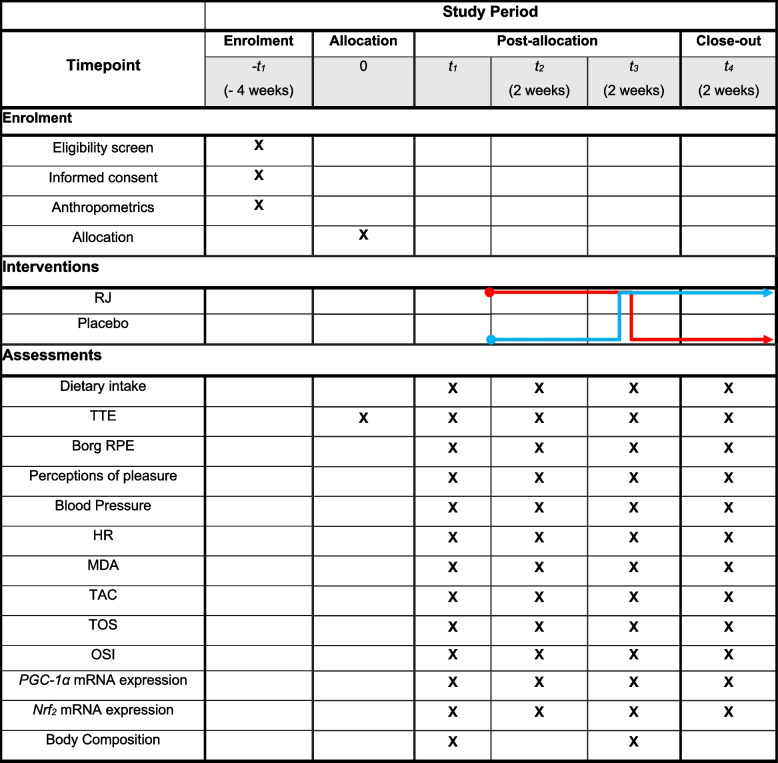
The study will use a 2 × 2 crossover design, integrating a 2-week washout period between the treatment phases (Fig. 1).
Fig. 1.
Schedule of enrolment, intervention, and assessment based on SPIRIT guidelines. The “X” refers to what is done in the given period. Abbreviations: RJ royal jelly, TTE time to exhaustion, RPE rating of perceived exertion, HR heart rate, MDA malondialdehyde, TAC total antioxidant capacity, TOS total oxidant capacity, OSI oxidative stress index, NRF2 nuclear factor erythroid 2-related factor 2, PGC-1α peroxisome proliferator-activated receptor gamma coactivator 1-alpha
Sample size {14}
The primary variable requiring the largest sample size was MDA. We calculated the sample size to ensure it would adequately capture all pertinent data. Considering a target difference of 1.4 nmol/mL (δ: effect size) and a standard deviation of 1.38 nmol/mL (σ) using the MDA index [21], with a two-tailed significance level of 0.05 (α), and statistical power of 80% (Zβ:1 − β), the sample size has been calculated to be 15 participants. Considering a dropout probability of 20% due to the longitudinal nature of the study, 18 individuals will initially be included in the study. If there are further dropouts during the study, sampling will continue until a minimum of 15 participants remain. The following formula was used to calculate the sample size [22]:
To ensure the statistical power of the study is preserved throughout its duration, a protocol has been established to replace participants. If an individual withdraws from the study for any reason, a new participant will be enrolled to maintain the balance between study conditions. This approach is designed to uphold the integrity of the study’s power and to safeguard the accuracy of the results obtained. These considerations were thoroughly evaluated before the initiation of the study to enhance the reliability and validity of the findings.
Recruitment {15}
Participants will be recruited from clubs in Kermanshah city, specifically targeting male individuals who have committed to regular endurance training—consisting of a minimum of three sessions per week—for at least 6 months before the study. Eligible athletes will be selected by a sports physiologist and nutritionist, and following an initial screening process, they will be randomly assigned to study conditions after providing their voluntary consent. Upon successful completion of participant enrolment, individuals will be randomly assigned to either the placebo condition or the RJ condition.
Assignment of interventions: allocation
Sequence generation {16a}
A computer-generated random number table will be utilized to establish the randomization sequence, with oversight provided by the study statistician. Each sequence will be documented on a card, sealed in opaque envelopes labeled with sequential numbers, and kept confidential until the enrolment phase. The envelopes will be opened in numerical order following the enrolment of eligible participants. The eligible participants will be randomly and blindly assigned in a 1:1 ratio to either the intervention or control conditions using simple randomization. Any participant excluded from the study will be replaced to maintain the overall sample size and statistical power.
Concealment mechanism {16b}
Sealed opaque envelopes labeled with the participants’ treatment identification codes will be used to conceal condition assignments from the investigators. Envelopes will be opened in numerical order following the enrollment of eligible participants. These participants will then be randomly and blindly assigned to either the intervention condition or the control condition in a 1:1 ratio (Fig. 2).
Fig. 2.
Trial design
Implementation {16c}
The study statistician will generate the allocation sequence, while M.M. will oversee participant enrollment and the assignment of participants to the appropriate interventions. As the principal investigator of the project, M.M. will manage the data, with support and guidance from our research team.
Assignment of interventions: blinding
Who will be blinded {17a}
The trial is a double-blinded study; the allocation of trial conditions will remain concealed from both participants and investigators—including those involved in enrollment, assessment, and analysis—until the study is completed and data analysis is finalized. A study statistician will have access to a randomization list, while sealed envelopes will be securely maintained throughout the trial. Furthermore, the study pharmacist will access sequentially numbered drug containers. All (RJ/placebo) capsules will be stored in identical numbered containers to maintain consistency and prevent bias. Additionally, the prepared capsules are uniformly labeled and contain contents that are visually indistinguishable in terms of shape, color, size, odor, and weight.
Procedure for unblinding if needed {17b}
If unblinding becomes necessary, such as in the case of a participant experiencing a complication, it will be carried out by the pharmacist.
Data collection and management
Plans for assessment and collection of outcomes {18a}
-
Demographic information
A face-to-face interview will collect comprehensive demographic information, including age, gender, education level, occupation, smoking habits, marital status, type and duration of sports participation, exercise history, allergies to bee products, dietary restrictions, musculoskeletal issues, supplement usage, medical history, and current medications.
-
2.
Nutritional assessments
Anthropometrics and body composition: Height will be measured to the nearest 0.5 cm using a stadiometer (DETECTO, Model 3PHTROD-WM, USA). BW, BMI, WHR, body fat percentage (BFP), and soft lean mass (SLM) will be determined using a bioelectric impedance body composition analyzer (Zeus 9.9 PLUS: Jawon Medical Co., Ltd., Kungsang Bukdo, South Korea). Dietary intake: To evaluate the dietary intake of participants throughout the study, they will be required to complete 3-day food record sheets, which include entries for two weekdays and one weekend day, at baseline as well as at the 2, 4, and 6 weeks. This approach is designed to minimize reliance on memory. In total, 12 food records will be collected and aggregated to determine the average dietary intake over the trial period. The gathered data will be analyzed using Nutritionist 4 software (First Databank, Hearst Corp, San Bruno, CA, USA), which has been modified for Iranian foods.
-
3.
The assessment of oxidative stress biomarkers
To assess antioxidant levels and gene expression, 5 cc of venous blood will be drawn from all participants before and after each treadmill test. The serum concentrations of MDA, TAC, and TOS will be measured using a commercial assay kit (Kiazist, Hamedan, Iran). For MDA assessment, a reaction with thiobarbituric acid at 95 °C will generate a colored complex that can be quantified at a wavelength of 540 nm. The concentration of MDA in serum will be determined using a standard curve, with results reported in nanomoles per milliliter (nmol/ml). TAC will be assessed by measuring the ability of antioxidants to reduce cupric ions (Cu2+) to cuprous ions (Cu+), resulting in a color change with the chromogen. Antioxidant levels will be evaluated through absorption measurements at 450 nm using a spectrophotometer, and serum TAC levels will be expressed as nanomoles of Trolox equivalent per milliliter, calculated against a Trolox standard curve. For TOS measurement, ferrous ions (Fe2+) are oxidized to ferric ions (Fe3+) in the presence of oxidants, leading to a color change with the chromogen. The absorbance, measured at 560 nm, will be directly correlated with the concentration of oxidants present. Additionally, we will calculate the oxidative stress index (OSI) using the formula: OSI (arbitrary unit) = TAC / TOS. This will provide a comprehensive insight into the balance between oxidative and antioxidative mechanisms in the participants.
-
4.
Incremental running test to measure MAS
To assess MAS [23], participants underwent an incremental running test on a treadmill during the enrolment visit, beginning with a warm-up followed by a speed increase of 1 km/h every 3 min. TTE was established by achieving at least 90% of the maximal predicted HR (calculated as 220 minus age) and a high perceived exertion rating of 19 or above on the Borg 20-6 scale. Participants refrained from specific activities before the test, and MAS was determined based on the final speed and duration. Men were considered endurance-trained if they could run the treadmill incremental test for at least 18 min.
MAS = V complete + (Inc × t/T).
V complete: speed at the completion of the last stage in the incremental test
Inc: speed increment from one stage to the next
t: time elapsed during an incomplete stage
T: time required and expected to complete a full-stage
-
5.
Endurance and exhaustive activity
Following the incremental test in the introductory session, participants will undergo an exhaustive test with increasing incline. Once exhaustion is reached in the incremental test, the final completed speed is noted, and 80% of the V peak (calculated using the formula provided) will be utilized in the aerobic test.
V peak = V complete + (T*Inc/t)
(V complete) = running speed at the last fully completed stage
(Inc) = speed increase (e.g., 1 km/h)
(t) = seconds spent in the incomplete stage
(T) = seconds needed to complete one stage
The researcher will monitor and offer feedback during the test, halting the activity if any issues arise. The treadmill settings for incline and speed will mirror those in the incremental test, except the participant will maintain the initial speed until exhaustion. If a participant cannot continue due to reaching 90% of their maximum HR or a Borg scale intensity of 19, the duration of the activity will be recorded as their personal best, known as the TTE parameter [23]. This test will take place on the H/P Cosmos Sports & Medical Pulsar 3p 4.0 treadmill, Germany.
-
6.
Gene expression assay
The rationale for evaluating the mRNA of Nrf2 and PGC-1α gene expression levels in this study stems from their significant roles in managing oxidative stress and enhancing athletic performance. Nrf2 is a crucial factor for regulating antioxidant proteins that protect against oxidative damage, which is particularly important for endurance athletes who undergo prolonged physical exertion [24]. Moreover, PGC-1α is a vital regulator factor of mitochondrial biogenesis and energy metabolism in skeletal muscle, contributing to improved endurance performance by promoting the formation of new mitochondria and enhancing muscle fiber composition [25]. By examining Nrf2 and PGC-1α gene expression levels, this trial seeks to uncover RJ’s mechanisms of action and its potential for improving athletic performance in endurance athletes and addressing these gaps by providing a comprehensive framework for studying RJ’s molecular impacts on endurance athletes.
Blood sample collection and peripheral blood mononuclear cell (PBMC) isolation: Blood samples will be obtained from individuals using sterile tubes containing heparin. To isolate serum, whole blood will be collected in a separate sterile microtube without anticoagulant. Peripheral blood mononuclear cells (PBMCs) will be separated using Ficoll. The blood sample will be mixed with phosphate buffer and placed on top of the Ficoll solution, followed by centrifugation at 600 × g for 20 min at 25 °C. PBMCs will be retrieved from the second layer (mononuclear cells) and washed three times with phosphate-buffered saline [26, 27].
Total RNA extraction: Total RNA will be extracted from PBMCs using TRIzol® reagent as per the manufacturer’s instructions. TRIzol® reagent and chloroform will be added to each sample tube. Following mixing and centrifugation, the upper aqueous phase will be transferred to a new tube. Isopropanol will be added, and the samples will be centrifuged to precipitate RNA. The RNA pellet will be washed with ethanol 70% and dissolved in diethyl pyrocarbonate-treated water. Subsequently, RNA will be reverse transcribed to cDNA utilizing the Easy ™ cDNA Synthesize Kit (ParsTous, Iran) and stored at −80 °C.
RT-qPCR assessment: Total RNA will be extracted from PBMCs using TRIzol® reagent as per the manufacturer’s guidelines. Subsequently, the RNA will undergo reverse transcription to cDNA with Master Mix. SYBR Green Master Mix will be utilized for quantifying mRNA expression levels on a Real-Time PCR System, following the manufacturer’s protocols. Target gene sequences were obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/), and specific primer sequences were meticulously designed manually and validated for accuracy using OLIGO 7 software. The primer details for target genes can be found in Table 1. The relative expression will be determined using the 2−ΔΔCT method [28]. Furthermore, melt curve analysis will be conducted to confirm the specificity of the amplified gene product. Finally, mRNA expression levels will be normalized to β-actin, serving as the housekeeping gene [26]. The expression levels of each gene in this study were measured in duplicate, and the average of the obtained data was calculated for gene expression evaluation. Also, real-time efficiency will be evaluated using the LinRegPCR software.
Table 1.
The sequences of the primers will be used in the RT-PCR reactions
| Gene | Forward | Reverse | Product size (bp) |
|---|---|---|---|
| Nrf-2 | CACATCCAGTCAGAAACCAGTG | CTACAAACGGGAATGTCTGCG | 121 |
| PGC1-α | ACCTACCGTTATACCTGTGA | TCCACAAAAGTACAGCTCAAA | 96 |
| β-actin | ATGACTTAGTTGCGTTACACC | AAACAAATAAAGCCATGCCAA | 84 |
Abbreviations: NRF-2 nuclear factor erythroid 2-related factor 2, PGC-1α peroxisome proliferator-activated receptor gamma coactivator 1-alpha, β-actin beta-actin, bp base pair, RT-PCR reverse transcription polymerase chain reaction
-
7.
Blood pressure and HR
The blood pressure assessment will occur 20 min after participants arrive at the laboratory. A digital sphygmomanometer (Beurer blood pressure monitor BM20, Germany) will measure blood pressure, with a systolic and diastolic accuracy of ±3 mmHg. Participants will sit with their feet on the ground and their arms resting at heart level. Blood pressure will be measured three times: before aerobic exercise, right after exercise, and after a 30-min rest to assess post-exercise hypotension [29]. HRs of participants will be monitored every 3 min using a Polar HR monitor (Finland) during the treadmill tests. The average HR for each experimental condition will subsequently be included in the statistical analysis.
-
8.
RPE assessment
We will utilize the Borg scale, ranging from 6 to 20, to evaluate RPE. Participants will record their RPE after each stage, at exhaustion during the incremental test, and at 3-min intervals, as well as at exhaustion in the time-to-exhaustion (TTE) test. These data points will be gathered to compute the mean RPE for each experimental condition, which will be later included in the statistical analysis [30].
-
9.
Felt pleasure and arousal
Participants’ emotional responses will be evaluated using a Feeling scale, an 11-point bipolar scale from −5 to +5, with higher values indicating increased pleasure levels [31]. During exercise, individuals may have different emotional states, with some enjoying it and others not. Emotions can also fluctuate throughout the exercise session. Participants will report their pleasure or displeasure every 3 min and post-exercise [32]. In addition, the Felt Arousal Scale (FAS), ranging from 1 (low arousal) to 6 (high arousal), will gauge arousal levels [33]. These pleasure and arousal responses will be noted by participants every 3 min during the activity and at exhaustion. The averaged data for each experimental condition will be part of the statistical analysis.
-
10.
Quality analysis and preparation of RJ
Fresh RJ was obtained from Negin Shahd Sepahan honey bee company in Isfahan, Iran. The quality of RJ, including its 10-hydroxy-2-decenoic acid (10-HDA) content, moisture, protein, fat, carbohydrates, and ash, was evaluated according to the standards of the Iranian National Standardization Organization (INSO). This analysis was conducted by the Isfahan Hourtash Laboratory. The School of Pharmacy at Mashhad University of Medical Sciences, Iran, is responsible for producing the RJ and placebo capsules. Each RJ capsule consists of RJ powder and starch as a filler, while the placebo capsules only contain starch. Both the RJ and placebo capsules will be carefully designed to be identical in size, color, shape, and flavor to maintain the blind design of the study.
Plans to promote participant retention and complete follow-up {18b}
The lead researcher will be readily available to offer guidance and advice to individuals via phone, email, and other communication channels.
Data management {19}
Data management adheres to the regulations of Kermanshah University of Medical Sciences. Data will be hosted on the DIGIT, a highly secure web-based data collection and storage platform (http://digit.kums.ac.ir). Throughout the project, we will enter and code data gathered during the recruitment, intervention, and evaluation phases into this platform using unique participant identifiers. After project termination, we will store data in a secure password-protected local drive. The IBM SPSS Statistics (version 22.0) and Stata (version 17) software will be utilized for comprehensive data analysis. Only authorized personnel will have access to the data, ensuring confidentiality and compliance with ethical standards.
Confidentiality {27}
Each participant will be assigned a unique identification code for research data, accessible only to researchers and safeguarded by the principal investigator during the trial. Personal details will remain confidential in all publications. Participants’ data will be securely stored at Kermanshah University, with access restricted to the trial investigators.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
A 5 ml blood sample will be collected before and after the treadmill test at each visit. Following collection, serum and buffy coat fractions will be separated through immediate centrifugation and stored at − 80 °C for subsequent analyses. The mRNA expression levels of Nrf2 and PGC1α will be quantified using reverse transcription polymerase chain reaction (RT-PCR). Additionally, serum TAC will be assessed using the CUPric Reducing Antioxidant Capacity method, TOS will be measured with a modified ferrous oxidation assay, and MDA levels will be determined via the thiobarbituric acid reactive substances (TBARS) method, utilizing commercial kits from Kiazist Life Sciences, Iran.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Data normality will be assessed using the Shapiro–Wilk test. Quantitative variables will be reported as mean ± standard deviation, while categorical data will be presented as frequencies and percentages. If the data are non-normal, transformation techniques such as logarithmic or square root transformations will be applied to achieve normality. If normality cannot be achieved, non-parametric tests will be employed. The primary statistical approach for this study is the linear mixed model (LMM), which is suitable for analyzing repeated measures and crossover study designs. The primary and secondary outcomes of this study will be analyzed using LMM and independent sample t-tests, as appropriate. An intention-to-treat (ITT) approach will be adopted to include all participants initially assigned to the study groups, regardless of their adherence or dropout status. This approach minimizes bias and preserves the study’s internal validity. Missing data for specific parameters will be excluded from the analysis of those parameters, but the overall ITT approach will be maintained. We do not anticipate a significant carryover effect due to the inclusion of a sufficient washout period and the random allocation of participants to different study sequences. Carryover effects will be evaluated using LMM assumptions in Stata, and additional checks for treatment, period, and sequence effects will be conducted in SPSS. LMM meets the necessary criteria for ITT analysis and provides robust statistical power for repeated measures and crossover designs, as supported by prior studies [34]. Data analysis will be conducted using Stata and SPSS software. The analyses will be performed by the primary researcher under the supervision of the study statistician. The LMM will include the intervention period (period 1 vs. period 2) and treatment order (placebo-RJ vs. RJ-placebo) as fixed effects. Participants will be treated as a random effect to account for inter-individual variability in treatment responses. Dietary intake, including specific antioxidants, will be incorporated as covariates to adjust for potential confounding factors.
Participants’ dietary intake will be monitored using 3-day food records, and these records will be used to adjust for confounders in the LMM. For the effects of RJ supplementation on TTE, HR, and RPE, adjustments will be made for changes in energy intake and antioxidant intake, specifically selenium, zinc, vitamin C, and vitamin E levels. When evaluating the effects of RJ supplementation on oxidative stress markers—such as MDA, TAC, TOS, and OSI—adjustments will include changes in TTE and dietary antioxidant levels. For the analysis of RJ supplementation’s impact on blood pressure, covariates will include dietary antioxidant levels, as well as sodium, potassium, and magnesium intake.
No P values will be reported for secondary outcomes; instead, these will be presented as differences in means and 95% confidence intervals. In the final analysis of the primary outcome, a two-tailed P value of less than 0.05 will be considered to indicate statistical significance.
Interim analyses {21b}
In this crossover clinical trial, an interim analysis will be performed to re-evaluate the sample size for all outcomes and assess the safety and efficacy of RJ supplementation. This analysis is crucial for enhancing the study’s efficiency, enabling us to adjust the sample size based on collected data and ensuring that the trial maintains adequate power, even if initial assumptions about within-person standard deviation are inaccurate [35, 36]. For the sample size re-estimation, we will involve an independent, unblinded statistician to maintain objectivity and ensure the integrity of the trial.
Methods for additional analyses (e.g., subgroup analyses) {20b}
All analyses are described in Sect. 20a.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
All primary analyses will employ an intention-to-treat (ITT) framework to ensure unbiased estimates of the intervention’s effectiveness, irrespective of participants’ adherence to the protocol. Participants will be analyzed based on their original randomization assignments, regardless of compliance or protocol deviations. Missing data will be carefully documented and classified. If the proportion of missing primary outcome data is less than 10%, the analysis will be considered a modified ITT (mITT) analysis. If the data are determined to be missing completely at random (MCAR) or missing at random (MAR), we will address this using Multiple Imputation by Chained Equations (MICE). This technique iteratively imputes missing values by accounting for the relationships between variables, ensuring valid statistical inference. The imputation model will include key baseline variables (e.g., age, sex, baseline outcome measures, and randomization condition) and any variables strongly correlated with missingness. For missing data classified as missing not at random (MNAR), targeted single imputation methods or sensitivity analyses will be conducted to assess the robustness of the results. These strategies aim to minimize bias resulting from dropouts or deviations from the study protocol while maintaining transparency in data handling.
Plans to give access to the full protocol, participant-level data, and statistical code (37)
The datasets from the trial will be accessible upon reasonable request. After the primary results are published, the trial protocol and statistical code will be made publicly available. Deidentified participant-level data may also be requested from the principal investigator following publication. Anonymized data will be provided for secondary analyses.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The researchers and all aspects of the study will be monitored by the Ethics Committee and the Vice-Chancellor of Kermanshah University of Medical Sciences. Their responsibility is to ensure adherence to ethical standards and to safeguard the health and dignity of the participants. In the event of any ethical breaches, appropriate corrective actions or termination of the study may be implemented. The principal investigator (PI) will act as the lead researcher and primary coordinator, overseeing participant recruitment, intervention, and follow-up. Furthermore, a steering committee (SC) will be established to oversee the entire study process, and the study team will engage in regular communication to track progress.
Composition of the data monitoring committee its role and reporting structure {21a}
The academic committee at Kermanshah University of Medical Sciences will conduct impartial and continuous monitoring of data. Any adverse events will be reported immediately and addressed accordingly. The Ethics Committee of Kermanshah University of Medical Sciences will maintain continuous oversight of the study, reviewing any safety issues or modifications to the protocol as necessary. This strategy is considered adequate to safeguard participant safety and maintain the integrity of the data throughout the study.
Patient and public involvement
While patients and the public were not directly involved in the initial design of this study, we recognize the importance of integrating their perspectives into research to enhance relevance and applicability. During the course of this trial, participants will have the opportunity to provide feedback on various aspects of the study, including the feasibility of the intervention, their experiences with the supplementation protocol, and the practical implications of the outcomes. This feedback will be systematically collected and considered during the analysis and dissemination phases to ensure the findings are meaningful and applicable to the athletic and broader public communities. Additionally, participant input will help inform the design of future studies with greater public involvement from the outset.
Adverse event reporting and harms {22}
Previous studies have demonstrated that RJ is safe and well tolerated, even at high doses, such as 20 g per day [37]. Additionally, another study found that consuming RJ at a dose of 3 g daily for 6 months is safe for healthy individuals [38]. Therefore, we do not anticipate any toxicities or significant side effects from the RJ supplementation of 1 g per day employed in this trial. If any complications arise during the RJ supplementation, the intervention will be promptly discontinued. The Data and Safety Monitoring Board (DSMB) will determine whether to unblind and exclude a participant from the study if the adverse effect requires medical attention. Necessary medical measures will be taken; costs for any required services will be covered; and the incident will be reported to the Ethics Committee of Kermanshah Medical University.
Frequency and plans for auditing trial conduct {23}
The current study will be overseen by the Ethics Committee of Kermanshah University of Medical Sciences. This committee will monitor the quality, validity, and compliance with ethical standards during at least two review sessions. Additionally, a report will be provided to the auditor every 3 months. The Project Management Group will convene monthly to assess the progress of the trial, tackle any arising issues, and ensure adherence to the protocol. Any deviations from the protocol or adverse events will be reported to the Ethics Committee immediately and addressed swiftly by the Project Management Group. These actions will facilitate consistent oversight and prompt identification of any concerns that could compromise the trial’s integrity.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
We outline a detailed plan for notifying changes to the protocol, starting with the funder, followed by the PI who will inform the centers. A revised protocol will be sent to the PI for inclusion in the Investigator Site File. Researchers will adhere to the protocol throughout the study. If any modifications occur—affecting study objectives, design, eligibility criteria, or sample sizes that could impact study conduct, benefits, or participant safety—they will also promptly inform the Vice President for Research and Technology at Kermanshah University of Medical Science and the ethics committee. Alterations to the study protocol, encompassing modifications to the sample size or methodological approaches, require prior approval from the Ethics Committee of Kermanshah University of Medical Sciences. Additionally, we will update the protocol in the Iranian Registry of Clinical Trials (https://irct.behdasht.gov.ir/). Any deviations from the protocol will be documented using a breach report form.
Dissemination plans {31a}
The conclusive results and data will be disclosed in upcoming publications.
Discussion
This protocol study explores the effects of RJ supplementation on oxidative stress markers, athletic performance, and the mRNA expression of Nrf2 and PGC-1α—key genes involved in mitochondrial biogenesis—in male endurance athletes. The study aims to address critical gaps in understanding RJ’s role in modulating ROS and physical performance. ROS, naturally produced during aerobic metabolism, can accumulate excessively during intense exercise, leading to oxidative stress that hinders muscle recovery and performance. RJ’s bioactive components, including flavonoids, peptides, and 10-HDA, exhibit antioxidant properties that may reduce ROS-induced damage by activating the Nrf2 signaling pathway [39]. This pathway regulates antioxidant enzymes such as superoxide dismutase and catalase, which help neutralize ROS and mitigate oxidative stress [40]. Moreover, research suggests RJ supplementation may lower markers of oxidative damage, such as lipid peroxidation, a significant contributor to exercise-induced muscle fatigue and tissue injury. For instance, a study by Ovchinnikov et al. demonstrated that RJ combined with coenzyme Q10 reduced oxidative stress biomarkers, including Schiff bases and dienoic conjugates, in swimmers after high-intensity interval training [41]. These findings emphasize RJ’s capacity to regulate ROS levels and maintain redox balance during and after exercise. Additionally, by upregulating PGC-1α, a critical regulator of mitochondrial biogenesis, RJ may enhance mitochondrial efficiency and support sustained energy production during prolonged activity. Increased mitochondrial density improves oxygen utilization and delays fatigue onset [42]. Also, RJ’s role in lactate metabolism could delay lactic acid accumulation in muscles, further supporting endurance. A recent systematic review highlighted the positive effects of RJ on lowering blood lactate levels and improving athletic performance [43]. In addition, the unique fatty acids in RJ, such as 10-HDA, can activate AMP-activated protein kinase (AMPK), an upstream regulator of PGC-1α, enhancing energy metabolism and resilience to oxidative stress [44]. RJ’s antioxidant and anti-inflammatory properties may also support cardiovascular health by lowering blood pressure, improving endothelial function, and enhancing oxygen delivery to muscles [45]. For instance, Araújo and Botelho noted improvements in cardiac autonomic regulation and reductions in exercise-induced tachycardia in athletes using RJ and coenzyme Q10 [46]. Despite promising findings, many studies lack detailed investigations into RJ’s molecular effects on oxidative stress and mitochondrial biogenesis. To our knowledge, this study is the pioneering trial to integrate gene expressions linked to mitochondrial biogenesis by examining Nrf2 and PGC-1α gene expression levels, oxidative stress biomarkers, and physiological assessments such as TTE as a measure of the aerobic performance of RJ in endurance athletes. This trial aims to address these gaps by providing a comprehensive framework for studying RJ’s molecular and physiological impacts of RJ on endurance athletes. If our findings support these theories, it will significantly progress our comprehension of RJ’s distinct functions and promote further clinical investigations. The findings may offer valuable insights into optimizing athletic nutrition and recovery strategies through the use of RJ.
Strengths
This study is the first clinical trial to investigate the effects of RJ supplementation on molecular and gene expression levels, oxidative stress biomarkers, and TTE in endurance athletes. Moreover, by employing a crossover design, this study effectively addresses the need to control for interindividual variability among participants. This design allows each participant to serve as their own control, thereby minimizing the impact of individual differences, such as baseline physical fitness levels, on the study outcomes. As a result, this methodological approach enhances the robustness of the findings, ensuring that the observed effects can be confidently attributed to the intervention rather than to inherent variations in participant characteristics.
Limitations
We acknowledge that the sample size is relatively small and was calculated based on a parallel trial design, while the current trial uses a crossover design. However, the crossover design inherently offers greater statistical efficiency by reducing within-subject variability, which likely increases the power of the study beyond the originally estimated 80%. Additionally, we were required to calculate the sample size based on MDA levels, as TAC levels required a larger sample size, due to the absence of prior research investigating the effects of RJ on TTE, TOS, and the gene expressions of PGC-1α and Nrf-2. Another limitation of this study is the use of simple randomization, which carries the inherent risk of baseline imbalances and unequal group sizes. Such imbalances could affect the comparability of the intervention and control conditions, potentially influencing the study’s outcomes. Although random assignment minimizes selection bias, it does not guarantee an equal distribution of important covariates in a small sample. Future studies with larger sample sizes should consider employing minimization procedures or stratified randomization methods to ensure better balance across groups for key baseline characteristics, thereby enhancing the validity of the findings.
Trial status
This trial was registered on https://irct.behdasht.gov.ir/ (IRCT20231209060310N1) on 2023 December 21 (the protocol version No: 1). Thus, it is considered to start on 2024 April 13 and will end on 10 July 2024.
Acknowledgements
We would like to thank the participants for their time and effort. We also gratefully acknowledge Kermanshah University of Medical Sciences and Razi University. Additionally, we extend our appreciation to the Student Research Committee at Kermanshah University of Medical Sciences, Kermanshah, Iran.
Abbreviations
- AMPK
AMP-activated protein kinase
- BFP
Body fat percentage
- BMI
Body mass index
- BW
Body weight
- DMC
Data monitoring committee
- DSMB
Data and Safety Monitoring Board
- FAS
Felt Arousal Scale
- 10-HAD
10-Hydroxy-2-decenoic acid
- HR
Heart rate
- INSO
Iranian National Standardization Organization
- ITT
Intention-to-treat
- LMM
Linear mixed model
- MAR
Missing at random
- MAS
Maximal aerobic speed
- MCAR
Missing completely at random
- MDA
Malondialdehyde
- MICE
Multiple Imputation by Chained Equations
- mITT
Modified intention-to-treat
- Nrf2
Nuclear factor erythroid 2-related factor 2
- OSI
Oxidative stress index
- PBMCs
Peripheral blood mononuclear cells
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI
Principal investigator
- RJ
Royal jelly
- ROS
Reactive oxygen species
- RPE
Ratings of perceived exertion
- RT-PCR
Reverse transcription polymerase chain reaction
- SC
Steering committee
- SLM
Soft lean mass
- TAC
Total antioxidant capacity
- TBARS
Thiobarbituric acid reactive substances
- TBW
Total body water
- TOS
Total oxidant status
- TTE
Time to exhaustion
- WHR
Waist-to-hip ratio
Authors’ contributions {31b}
MM, VT, and YP wrote the manuscript and coordinated the study. ES, MA, and AS are the investigators; they designed the study and will interpret the results. MM and FN will analyze and interpret the results and will provide statistical advice. All authors edited and approved the final manuscript.
Funding {4}
The study is financially supported by Kermanshah University of Medical Sciences (grant number: 4020784); however, the funder is not engaged in study design, data analysis, manuscript writing, or publication decisions.
Data availability {29}
Any data required to support the protocol will be supplied on reasonable request by the corresponding author.
Declarations
Ethics approval and consent to participate {24}
This trial was approved by the Ethics Committee of Kermanshah University of Medical Sciences with code No. IR.KUMS.REC.1402.419 and conducted in accordance with the Declaration of Helsinki. Moreover, it was registered in the Iranian Registry of Clinical Trials (https://www.irct.ir) on 21 December 2023 with the code number IRCT20231209060310N1. Written informed consent to participate will be obtained from all participants.
Consent for publication {32}
Not applicable.
Competing interests {28}
The authors declare that they do not have competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anugrah SM, Kusnanik NW, Wahjuni ES, Ayubi N, Mulyawan R. Effect of royal jelly on performance and inflammatory response to muscle damage: a systematic review. Biointerface Research in Applied Chemistry. 2023;13(5):6–13. [Google Scholar]
- 2.Thomas DT, Erdman KA, Burke LM. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–68. [DOI] [PubMed] [Google Scholar]
- 3.Amawi A, AlKasasbeh W, Jaradat M, Almasri A, Alobaidi S, Hammad AA, et al. Athletes' nutritional demands: a narrative review of nutritional requirements. Front Nutr. 2023;10:1331854. [DOI] [PMC free article] [PubMed]
- 4.Rani Jose RJ, Usha Chandrasekhar UC. Nutritional knowledge, attitude and practices of selected sports men and women in Hyderabad. 2010.
- 5.Wickham KA, Spriet LL. Food for thought: physiological considerations for nutritional ergogenic efficacy. Scand J Med Sci Sports. 2024;34(1): e14307. [DOI] [PubMed] [Google Scholar]
- 6.Braakhuis A, Somerville V, Hurst R. The effect of New Zealand blackcurrant on sport performance and related biomarkers: a systematic review and meta-analysis. J Int Soc Sports Nutr. 2020;17(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G. The positive and negative aspects of reactive oxygen species in sports performance. Current issues in sports and exercise medicine. 2013.
- 8.Clemente-Suárez VJ, Bustamante-Sanchez Á, Mielgo-Ayuso J, Martínez-Guardado I, Martín-Rodríguez A, Tornero-Aguilera JF. Antioxidants and sports performance. Nutrients. 2023;15(10): 2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirupathi A, Pinho RA. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J Physiol Biochem. 2018;74:359–67. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Wang Y, Deng S, Lian Z, Yu K. Skeletal muscle oxidative stress and inflammation in aging: focus on antioxidant and anti-inflammatory therapy. Frontiers in Cell and Developmental Biology. 2022;10: 964130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayacan Y, Yazar H. Oxidative stress biomarkers in exercise: intake of supplements. Biomarkers in nutrition: Springer; 2022. p. 819–31.
- 12.Kruk J, Aboul-Enein BH, Duchnik E, Marchlewicz M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J Physiol Sci. 2022;72(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peeling P, Castell LM, Derave W, de Hon O, Burke LM. Sports foods and dietary supplements for optimal function and performance enhancement in track-and-field athletes. Int J Sport Nutr Exerc Metab. 2019;29(2):198–209. [DOI] [PubMed] [Google Scholar]
- 14.Ghazzawi HA, Hussain MA, Raziq KM, Alsendi KK, Alaamer RO, Jaradat M, et al. Exploring the relationship between micronutrients and athletic performance: a comprehensive scientific systematic review of the literature in sports medicine. Sports. 2023;11(6): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ovchinnikov AN, Deryugina AV, Paoli A. Royal jelly plus coenzyme q10 supplementation enhances high-intensity interval exercise performance via alterations in cardiac autonomic regulation and blood lactate concentration in runners. Front Nutr. 2022;9: 893515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mofid B, Rezaeizadeh H, Termos A, Rakhsha A, Mafi AR, Taheripanah T, et al. Effect of processed honey and royal jelly on cancer-related fatigue: a double-blind randomized clinical trial. Electron Physician. 2016;8(6):2475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vajdi M, Musazadeh V, Khajeh M, Safaei E, Darzi M, Noshadi N, et al. The effects of royal jelly supplementation on anthropometric indices: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Front Nutr. 2023;10: 1196258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahari H, Taheri S, Rashidmayvan M, Hezaveh ZS, Mousavi SE, Malekahmadi M. The effects of royal jelly consumption on lipid profile: a GRADE-assessed systematic review and dose-response meta-analysis. PharmaNutrition. 2023;25: 100351. [Google Scholar]
- 19.Liang Y, Kagota S, Maruyama K, Oonishi Y, Miyauchi-Wakuda S, Ito Y, et al. Royal jelly increases peripheral circulation by inducing vasorelaxation through nitric oxide production under healthy conditions. Biomed Pharmacother. 2018;106:1210–9. [DOI] [PubMed] [Google Scholar]
- 20.Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–9. [DOI] [PubMed] [Google Scholar]
- 21.Pourmoradian S, Mahdavi R, Mobasseri M, Faramarzi E, Mobasseri M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: a randomized clinical trial. Chin J Integr Med. 2014;20:347–52. [DOI] [PubMed] [Google Scholar]
- 22.Sakpal TV. Sample size estimation in clinical trial. Perspect Clin Res. 2010;1(2):67–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Machado FA, Kravchychyn ACP, Peserico CS, da Silva DF, Mezzaroba PV. Incremental test design, peak ‘aerobic’ running speed and endurance performance in runners. J Sci Med Sport. 2013;16(6):577–82. [DOI] [PubMed] [Google Scholar]
- 24.Ngo V, Duennwald ML. Nrf2 and oxidative stress: a general overview of mechanisms and implications in human disease. Antioxidants. 2022;11(12): 2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE. 2011;6(12): e28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Cai D, Zhong H, Liu F, Jiang Q, Liang J, et al. Mitochondrial dynamics and biogenesis indicators may serve as potential biomarkers for diagnosis of myasthenia gravis. Exp Ther Med. 2022;23(4):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Y, Xu H, Li Y, Wei C, Guo R, Wang F, et al. A modified ficoll-paque gradient method for isolating mononuclear cells from the peripheral and umbilical cord blood of humans for biobanks and clinical laboratories. Biopreservation and biobanking. 2018;16(2):82–91. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8. [DOI] [PubMed]
- 29.Kotte EM, De Groot JF, Bongers BC, Winkler AM, Takken T. Validity and reproducibility of a new treadmill protocol: the Fitkids Treadmill Test. Med Sci Sports Exerc. 2015;47(10):2241–7. [DOI] [PubMed] [Google Scholar]
- 30.Okano AH, Fontes EB, Montenegro RA, Farinatti PdTV, Cyrino ES, Li LM, et al. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. British journal of sports medicine. 2015;49(18):1213–8. [DOI] [PubMed] [Google Scholar]
- 31.Astorino TA, Cottrell T, Lozano AT, Aburto-Pratt K, Duhon J. Effect of caffeine on RPE and perceptions of pain, arousal, and pleasure/displeasure during a cycling time trial in endurance trained and active men. Physiol Behav. 2012;106(2):211–7. [DOI] [PubMed] [Google Scholar]
- 32.Hardy CJ, Rejeski WJ. Not what, but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol. 1989;11(3):304–17. [Google Scholar]
- 33.Rodrigues GM, Machado S, Vieira LAF, de Oliveira BRR, Abreu MAJ, Marquez G, et al. Effects of anodal transcranial direct current stimulation on training volume and pleasure responses in the back squat exercise following a bench press. The Journal of Strength & Conditioning Research. 2022;36(11):3048–55. [DOI] [PubMed] [Google Scholar]
- 34.Praet SFE, Purdam CR, Welvaert M, Vlahovich N, Lovell G, Burke LM, et al. Oral supplementation of specific collagen peptides combined with calf-strengthening exercises enhances function and reduces pain in Achilles tendinopathy patients. Nutrients. 2019;11(1): 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook RJ. Interim analyses in 2 x 2 crossover trials. Biometrics. 1995:932–45. [PubMed]
- 36.Cook RJ, Willan AR. Design considerations in crossover trials with a single interim analysis and serial patient entry. Biometrics. 1996:732–9. [PubMed]
- 37.Münstedt K, Bargello M, Hauenschild A. Royal jelly reduces the serum glucose levels in healthy subjects. J Med Food. 2009;12(5):1170–2. [DOI] [PubMed] [Google Scholar]
- 38.Morita H, Ikeda T, Kajita K, Fujioka K, Mori I, Okada H, et al. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr J. 2012;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Botezan S, Baci G-M, Bagameri L, Pașca C, Dezmirean DS. Current status of the bioactive properties of royal jelly: a comprehensive review with a focus on its anticancer, anti-inflammatory, and antioxidant effects. Molecules. 2023;28(3): 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ovchinnikov AN, Paoli A, Seleznev VV, Deryugina AV. Royal jelly plus coenzyme Q10 supplementation improves high-intensity interval exercise performance via changes in plasmatic and salivary biomarkers of oxidative stress and muscle damage in swimmers: a randomized, double-blind, placebo-controlled pilot trial. J Int Soc Sports Nutr. 2022;19(1):239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi Y, Hijikata K, Seike K, Nakano S, Banjo M, Sato Y, et al. Effects of royal jelly administration on endurance training-induced mitochondrial adaptations in skeletal muscle. Nutrients. 2018;10(11): 1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasdar Y, Tadibi V, Sadeghi E, Najafi F, Miryan M. The impact of royal jelly on athletic performance, lactate levels, anthropometric parameters, and muscle damage: a systematic review: Prev Nutr Food Sci. 2024 Dec 31;29(4):385-93. 10.3746/pnf.2024.29.4.385. Epub 2024 Dec 31 [DOI] [PMC free article] [PubMed]
- 44.Takikawa M, Kumagai A, Hirata H, Soga M, Yamashita Y, Ueda M, et al. 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5′-AMP-activated protein kinase in L6 myotubes and mice. Mol Nutr Food Res. 2013;57(10):1794–802. [DOI] [PubMed] [Google Scholar]
- 45.Oršolić N, Jazvinšćak JM. Royal jelly: biological action and health benefits. Int J Mol Sci. 2024;25(11):6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araújo MM, Botelho PB. Probiotics, prebiotics, and synbiotics in chronic constipation: outstanding aspects to be considered for the current evidence. Front Nutr. 2022;9: 935830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data required to support the protocol will be supplied on reasonable request by the corresponding author.