Abstract
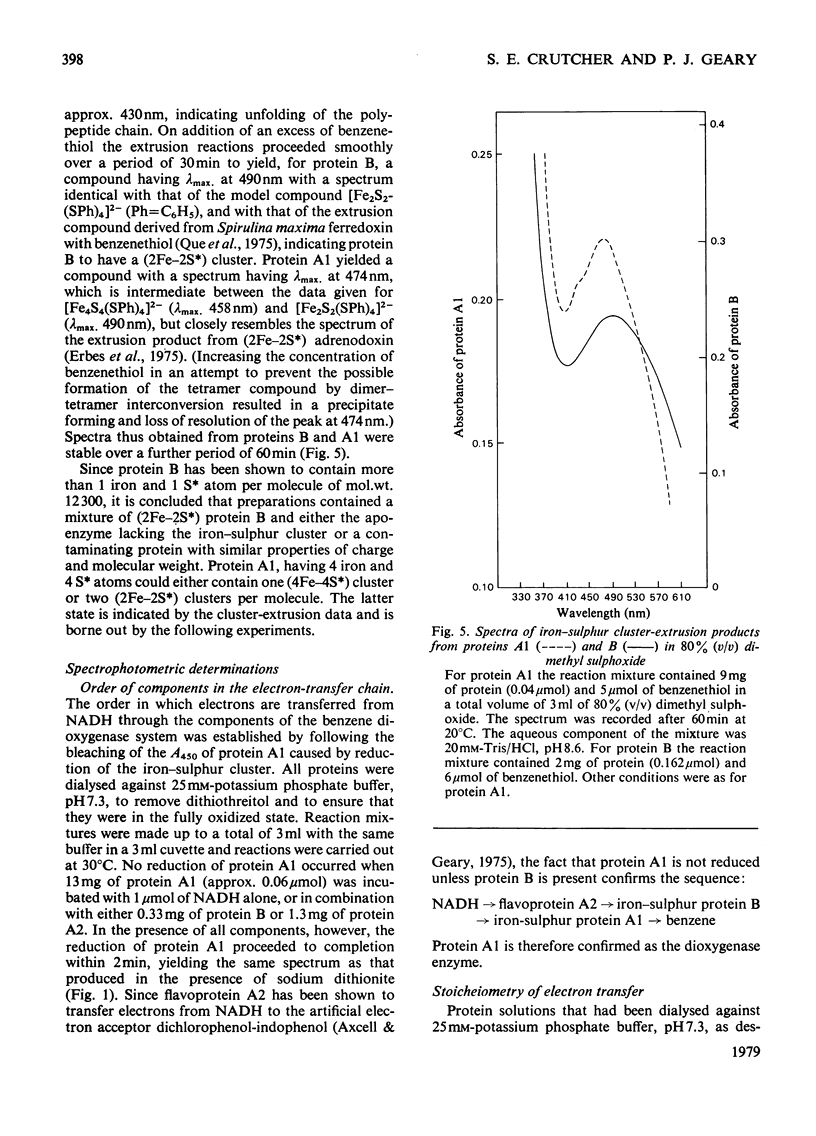
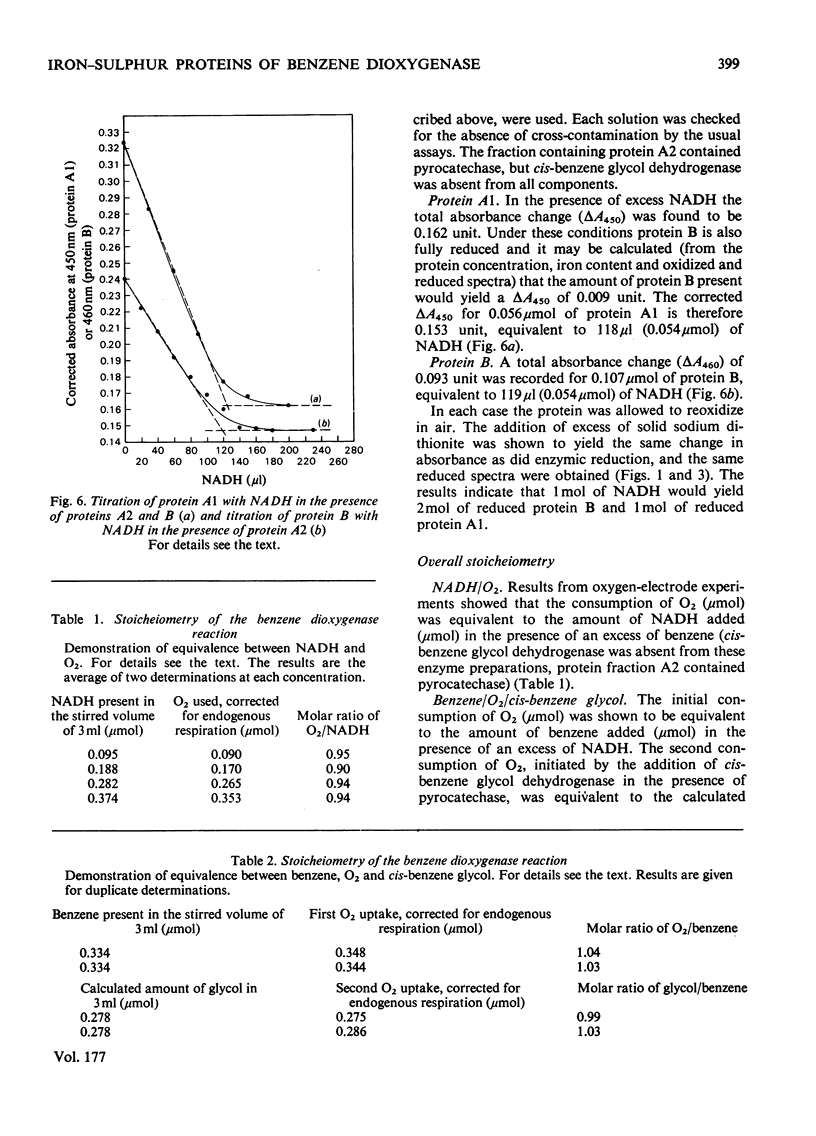
A purification procedure was developed to stabilize the iron-sulphur proteins of the benzene dioxygenase system from Pseudomonas putida. The intermediate electron-carrying protein has a mol. wt. of 12300 and possesses one (2Fe--2S) cluster, whereas the terminal dioxygenase has a mol.wt. of 215300 and possesses two (2Fe--2S) clusters. The order and stoicheiometry of electron transfer and of the whole system are described.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. The metabolism of benzene by bacteria. Purification and some properties of the enzyme cis-1,2-dihydroxycyclohexa-3,5-diene (nicotinamide adenine dinucleotide) oxidoreductase (cis-benzene glycol dehydrogenase). Biochem J. 1973 Dec;136(4):927–934. doi: 10.1042/bj1360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenathan T., Akagi J. M., Hersh R. T., Himes R. H. Ferredoxin from two thermophilic clostridia. J Biol Chem. 1969 Jun 10;244(11):2846–2853. [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H., Orme-Johnson W. H. On the iron-sulfur cluster in hydrogenase from Clostridium pasteurianum W5. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4795–4799. doi: 10.1073/pnas.72.12.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R. H. Iron-sulphur clusters in natural and synthetic systems. Endeavour. 1975 Jan;34(121):38–43. doi: 10.1016/0160-9327(75)90067-8. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Que L., Jr, Holm R. H., Mortenson L. E. Letter: Extrusion of Fe2S2 and Fe4S4 cores from the active sites of ferredoxin proteins. J Am Chem Soc. 1975 Jan 22;97(2):463–464. doi: 10.1021/ja00835a064. [DOI] [PubMed] [Google Scholar]
- Sauber K., Fröhner C., Rosenberg G., Eberspächer J., Lingens F. Purification and properties of pyrazon dioxygenase from pyrazon-degrading bacteria. Eur J Biochem. 1977 Mar 15;74(1):89–97. doi: 10.1111/j.1432-1033.1977.tb11370.x. [DOI] [PubMed] [Google Scholar]
- Stombaugh N. A., Sundquist J. E., Burris R. H., Orme-Johnson W. H. Oxidation-reduction properties of several low potential iron-sulfur proteins and of methylviologen. Biochemistry. 1976 Jun 15;15(12):2633–2641. doi: 10.1021/bi00657a024. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M., Wada K., Kobayashi H. Estimation of labile sulfide in iron-sulfur proteins. Anal Biochem. 1975 Oct;68(2):632–636. doi: 10.1016/0003-2697(75)90659-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yeh W. K., Gibson D. T., Liu T. N. Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun. 1977 Sep 9;78(1):401–410. doi: 10.1016/0006-291x(77)91268-2. [DOI] [PubMed] [Google Scholar]
- Zabinski R., Münck E., Champion P. M., Wood J. M. Kinetic and Mössbauer studies on the mechanism of protocatechuic acid 4,5-oxygenase. Biochemistry. 1972 Aug 15;11(17):3212–3219. doi: 10.1021/bi00767a012. [DOI] [PubMed] [Google Scholar]