Abstract
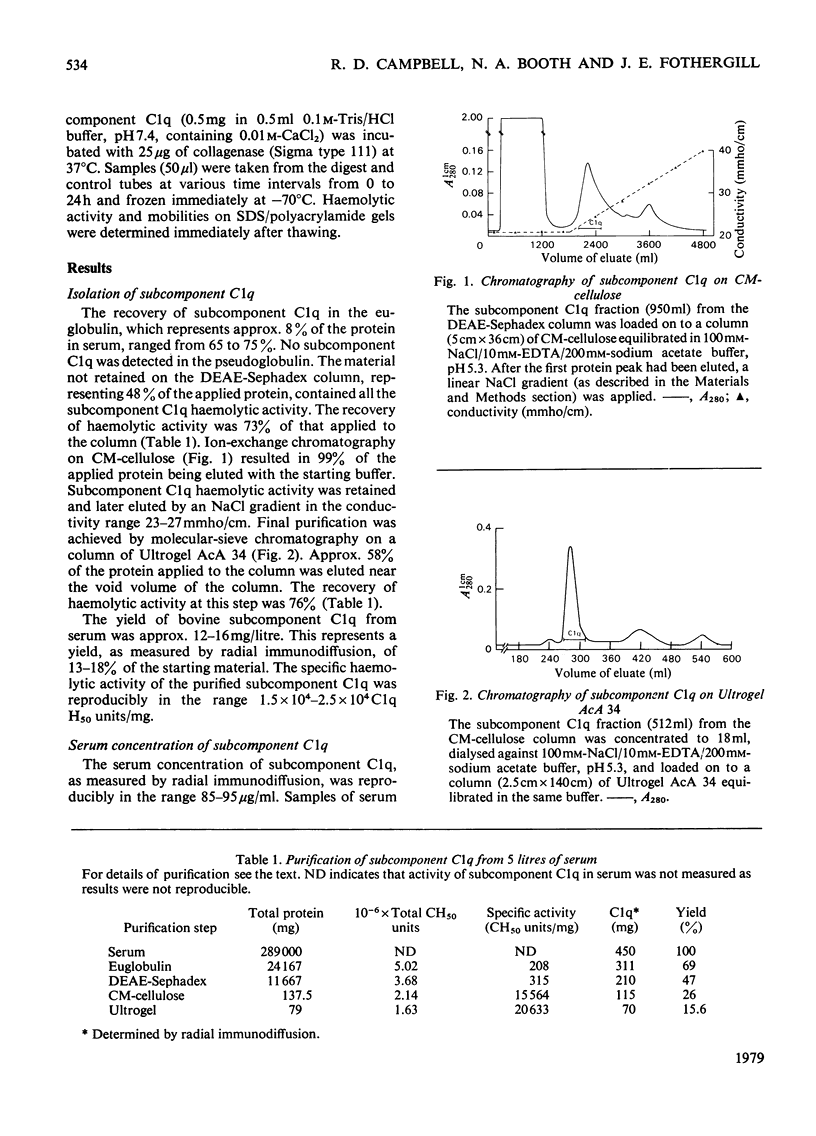
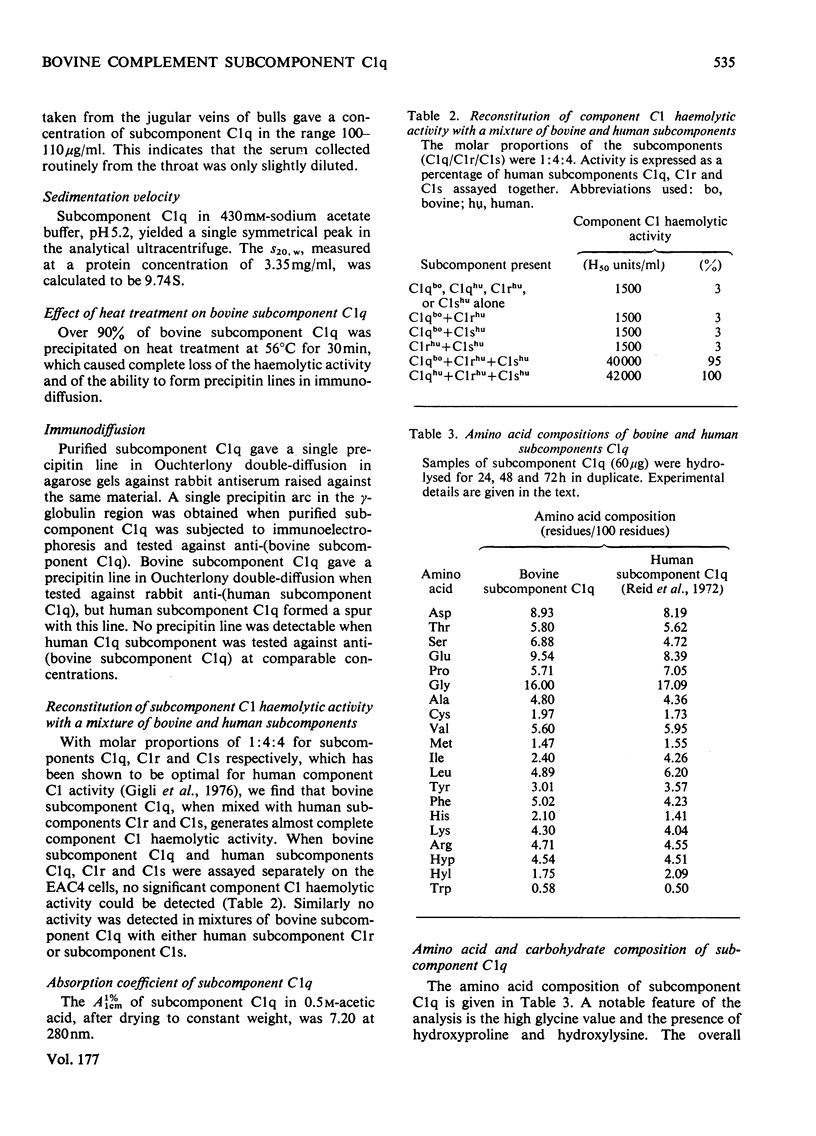
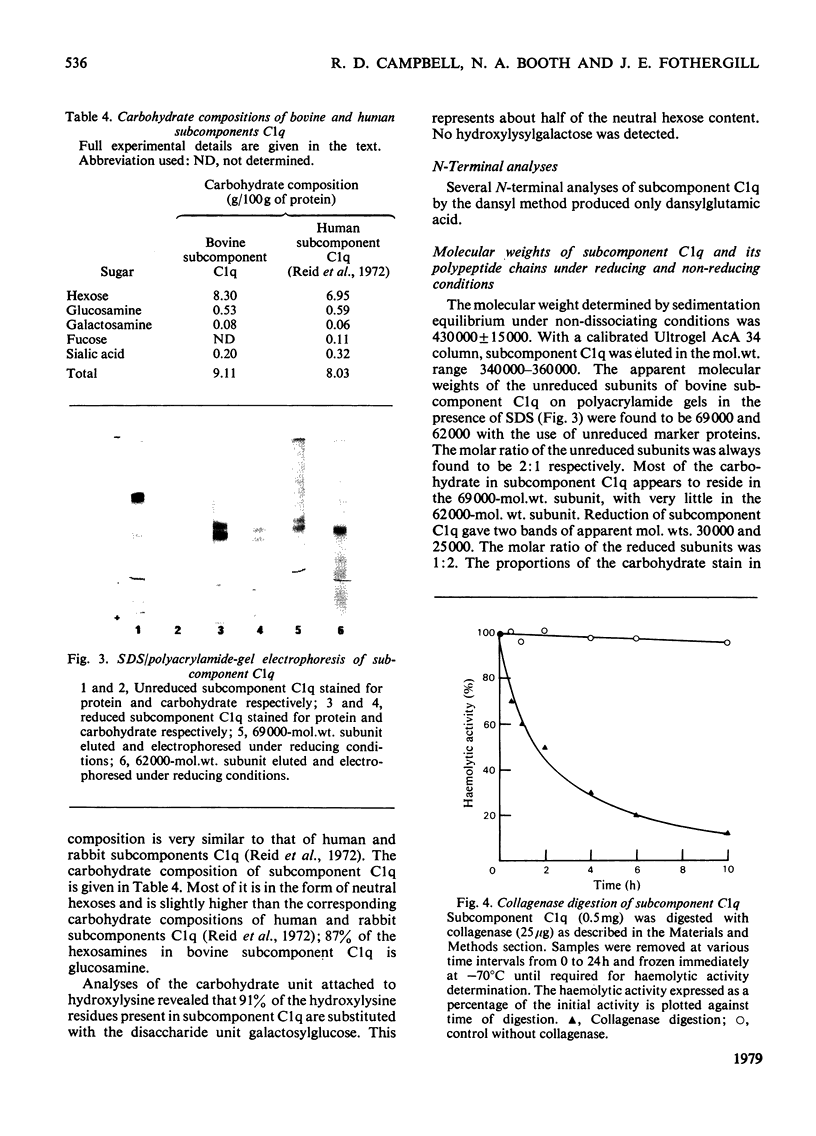
Bovine C1q, a subcomponent of the first component of complement, was purified in high yield by a combination of euglobulin precipitation, and ion-exchange and molecularsieve chromatography on CM-cellulose and Ultrogel AcA 34. Approx. 12–16mg can be isolated from 1 litre of serum, representing a yield of 13–18%. The molecular weight of undissociated subcomponent C1q, as determined by equilibrium sedimentation, is 430000. On sodium dodecyl sulphate/polyacrylamide gels under non-reducing conditions, subcomponent C1q was shown to consist of two subunits of mol.wts. 69000 and 62000 in a molar ratio of 2:1. On reduction, the 69000-mol.wt. subunit gave chains of mol.wts. 30000 and 25000 in equimolar ratio, and the 62000-mol.wt. subunit decreased to 25000. The amino acid composition, with a high value for glycine, and the presence of hydroxyproline and hydroxylysine, suggests that there is a region of collagen-like sequence in the molecule. This is supported by the loss of haemolytic activity and the degradation of the polypeptide chains of subcomponent C1q when digested by collagenase. All of these molecular characteristics support the structure of six subunits, each containing three different polypeptide chains, with globular heads connected by collagen triple helices as proposed by Reid & Porter (1976) (Biochem. J. 155, 19–23) for human subcomponent C1q. Subcomponent C1q contains approx. 9% carbohydrate; analysis of the degree of substitution of the hydroxylysine residues revealed that 91% are modified by the addition of the disaccharide unit Gal-Glc. Bovine subcomponent C1q generates full C1 haemolytic activity when assayed with human subcomponents C1r and C1s.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Barta O., Barta V. Haemolytic assay of bovine serum complement. J Immunol Methods. 1972 Aug;1(4):363–374. doi: 10.1016/0022-1759(72)90029-4. [DOI] [PubMed] [Google Scholar]
- Calcott M. A., Müller-Eberhard H. J. C1q protein of human complement. Biochemistry. 1972 Aug 29;11(18):3443–3450. doi: 10.1021/bi00768a018. [DOI] [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J. S., Muschel L. H., Good R. A. Kinetics of bovine complement. I. Formation of a lytic intermediate. J Immunol. 1971 Jul;107(1):28–33. [PubMed] [Google Scholar]
- Fong J. S., Muschel L. H., Good R. A. Kinetics of bovine complement. II. Properties of the lytic intermediate. J Immunol. 1971 Jul;107(1):34–40. [PubMed] [Google Scholar]
- Fothergill J. E., Anderson W. H. A molecular approach to the complement system. Curr Top Cell Regul. 1978;13:259–311. doi: 10.1016/b978-0-12-152813-3.50012-4. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factors X 1 and X 2 (Stuart factor). Isolation and characterization. Biochemistry. 1972 Dec 19;11(26):4882–4891. doi: 10.1021/bi00776a002. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isemura M., Takahashi K., Ikenaka T. Comparative study of carbohydrate-protein complexes. II. Determination of hydroxylysine and its glycosides in human skin and scar collagens by an improved method. J Biochem. 1976 Oct;80(4):653–658. doi: 10.1093/oxfordjournals.jbchem.a131324. [DOI] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Davie E. W. Isolation and characterization of bovine factor VII. Biochemistry. 1975 Nov 4;14(22):4928–4934. doi: 10.1021/bi00693a023. [DOI] [PubMed] [Google Scholar]
- Knobel H. R., Heusser C., Rodrick M. L., Isliker H. Enzymatic digestion of the first component of human complement (C1q). J Immunol. 1974 Jun;112(6):2094–2101. [PubMed] [Google Scholar]
- Knobel H. R., Villiger W., Isliker H. Chemical analysis and electron microscopy studies of human C1q prepared by different methods. Eur J Immunol. 1975 Jan;5(1):78–82. doi: 10.1002/eji.1830050119. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowe D. M., Reid K. B. Studies on the structure and activity of rabbit Clq (a subcomponent of the first component of complement). Biochem J. 1974 Nov;143(2):265–272. doi: 10.1042/bj1430265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- RICE C. E., CROWSON C. N. The interchangeability of the complement components of different animal species in the hemolysis of sheep erythrocytes sensitized with rabbit amboceptor. J Immunol. 1950 Aug;65(2):201–210. [PubMed] [Google Scholar]
- Reid K. B. A collagen-like amino acid sequence in a polypeptide chain of human C1q (a subcomponent of the first component of complement). Biochem J. 1974 Jul;141(1):189–203. doi: 10.1042/bj1410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Amino acid sequence of the N-terminal forty-two amino acid residues of the C chain of subcomponent C1q of the first component of human complement. Biochem J. 1977 Feb 1;161(2):247–251. doi: 10.1042/bj1610247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent Clq of the first component of human complement. Biochem J. 1976 Apr 1;155(1):5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. Subunit composition and structure of subcomponent C1q of the first component of human complement. Biochem J. 1976 Apr 1;155(1):19–23. doi: 10.1042/bj1550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Schmer G., Kirby E. P., Teller D. C., Davie E. W. The isolation nd characterization of bovine factor VIII (antihemophilic factor). J Biol Chem. 1972 Apr 25;247(8):2512–2521. [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanakis J. E., Stroud R. M. Rabbit C1q: purification, functional and structural studies. J Immunol Methods. 1972 Nov;2(1):25–34. doi: 10.1016/0022-1759(72)90014-2. [DOI] [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Yasmeen D., Ellerson J. R., Dorrington K. J., Painter R. H. The structure and function of immunoglobulin domains. IV. The distribution of some effector functions among the Cgamma2 and Cgamma3 homology regions of human immunoglobulin G1. J Immunol. 1976 Feb;116(2):518–526. [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M. Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J Immunol. 1971 Feb;106(2):304–313. [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M., Niedermeier W., Butler W. T. Chemical studies of Clq; a modulator of immunoglobulin biology. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1388–1394. doi: 10.1016/s0006-291x(71)80028-1. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]