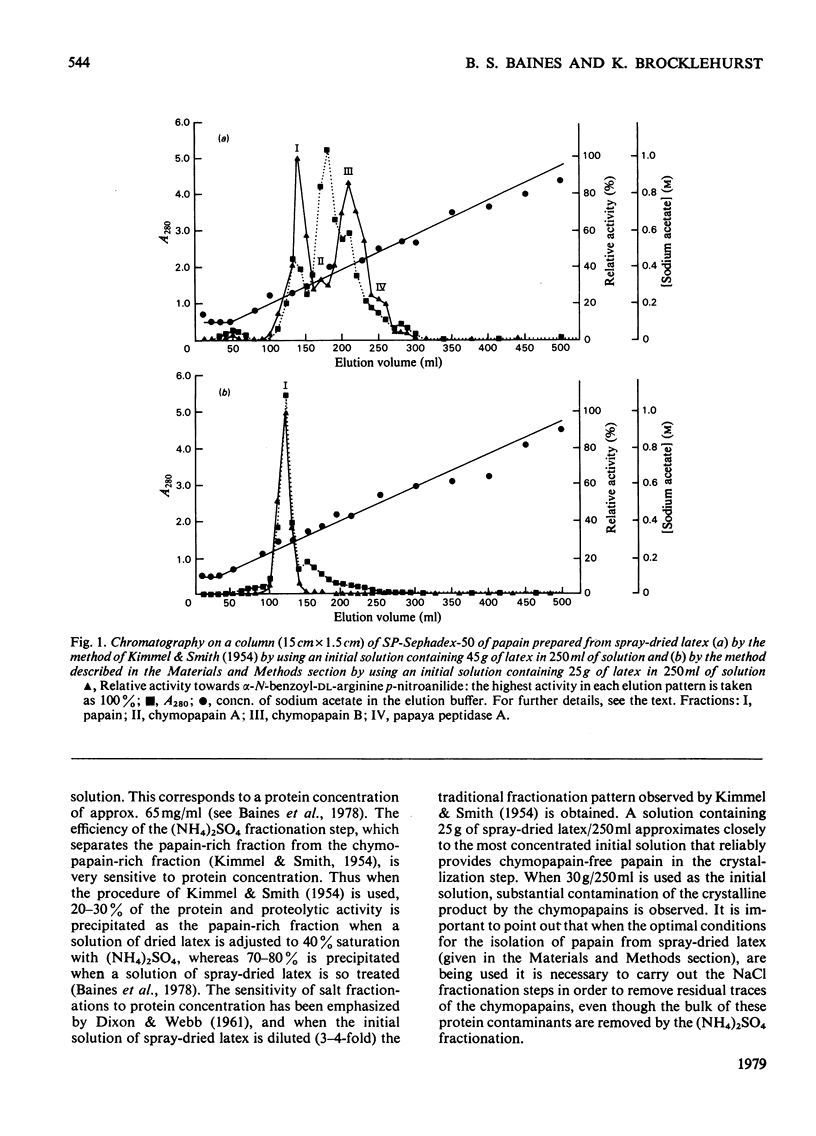
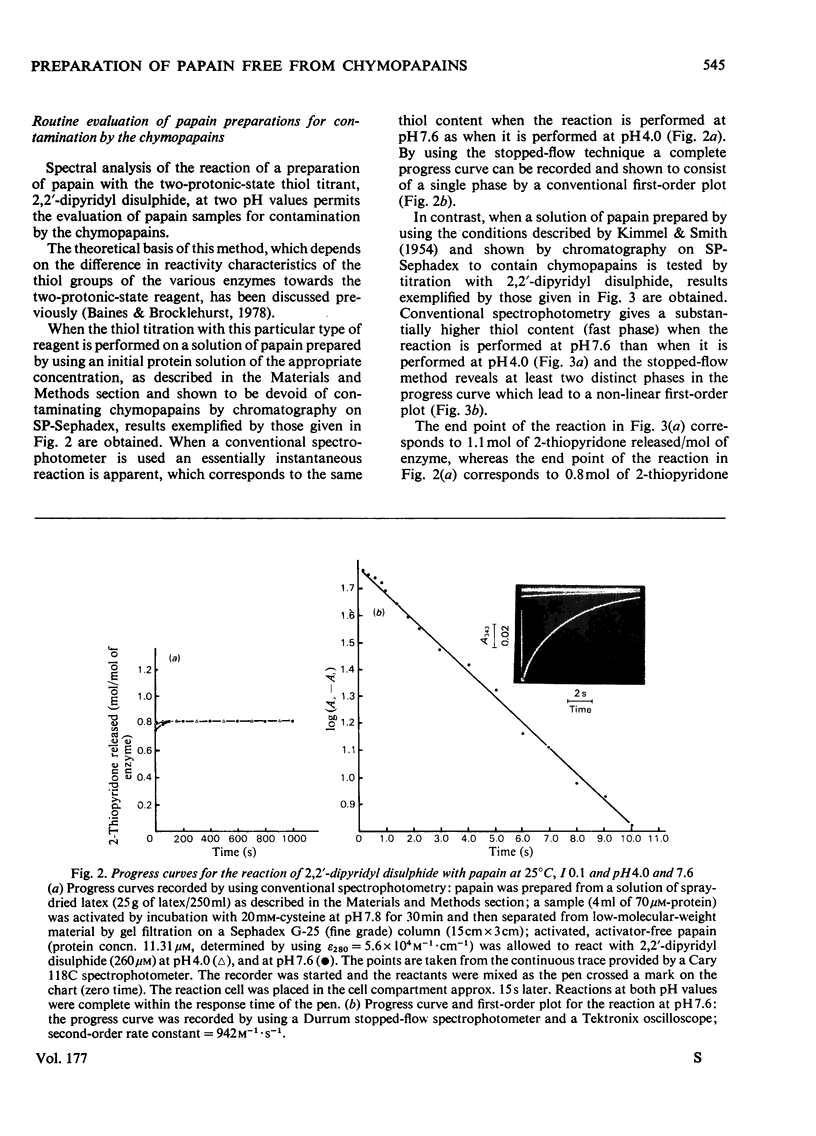
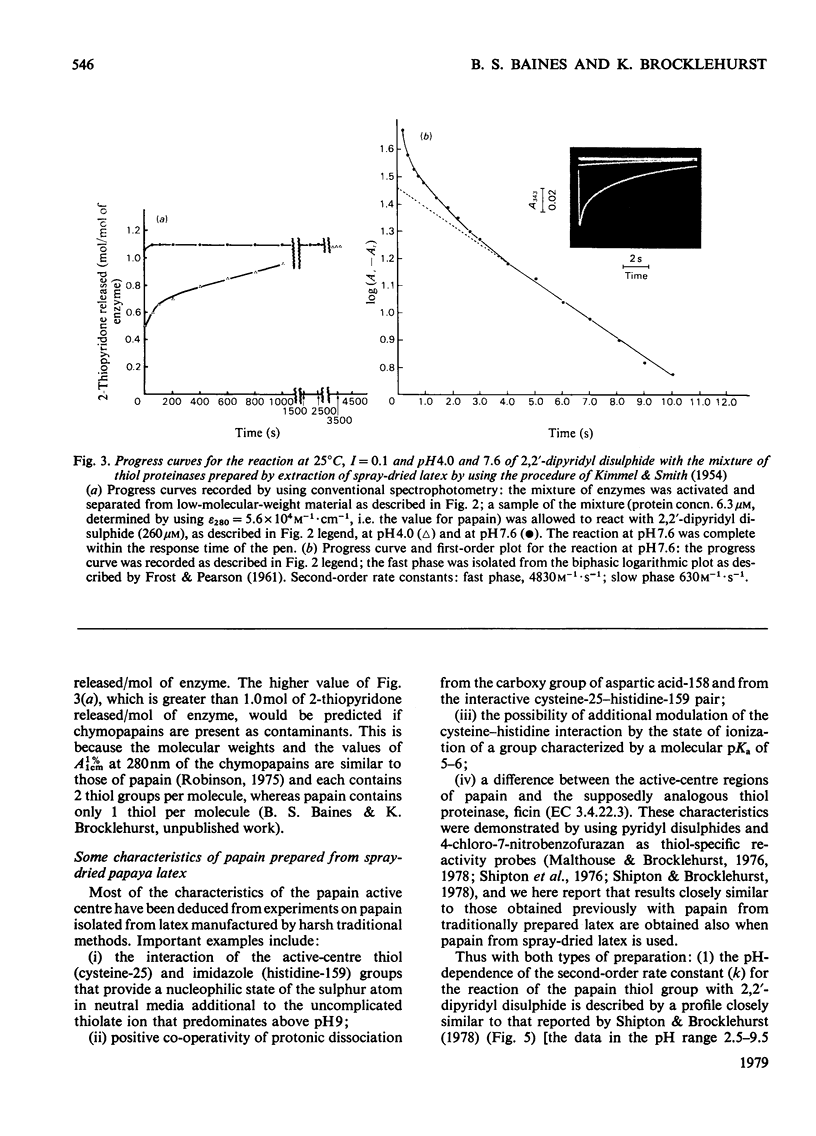
Abstract
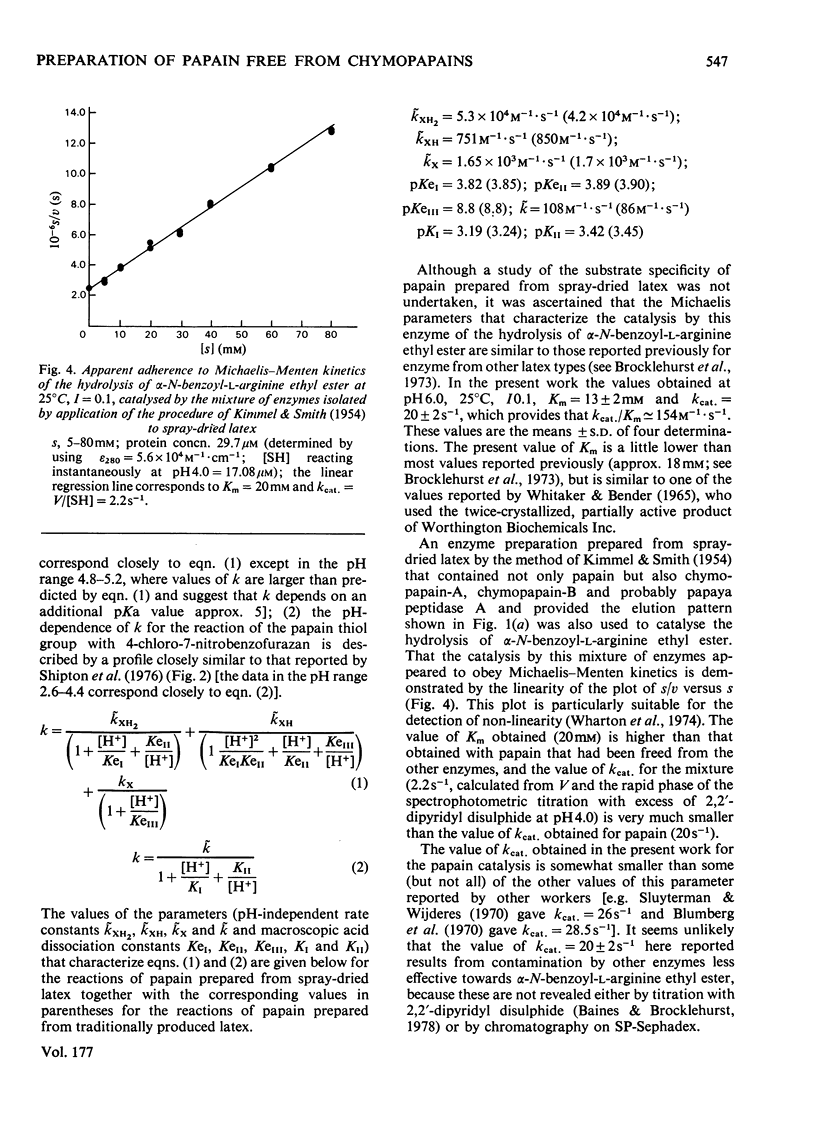
A method of preparation of papain (EC 3.4.22.2) from relatively soluble types of latex of Carica papaya, including spray-dried latex produced by a controlled and relatively mild process, was devised. Spray-dried latex dissolves easily in water up to 350mg/ml at 22°C, which corresponds to approx. 230mg of protein/ml. When the usual method of preparation of crystalline papain contaminated only by its oxidation products, developed by Kimmel & Smith [J. Biol. Chem. (1954) 207, 515–531], is applied to spray-dried latex, the result is a preparation of papain heavily contaminated by chymopapains A and B (EC 3.4.22.6), and to a lesser extent by papaya peptidase A. This applies also to other types of papaya-latex currently commercially available, which, though less soluble than spray-dried latex, are more soluble than the types of latex available when the method of Kimmel & Smith (1954) was developed. This contamination is avoided by adjusting the concentration of the initial latex extract to 65mg of protein/ml (or less) before salt fractionation. For spray-dried latex this corresponds to 100mg of latex/ml. Papain isolated from spray-dried latex was characterized by using 2,2′-dipyridyl disulphide and 4-chloro-7-nitrobenzofurazan as thiol-specific reactivity probes and α-N-benzoyl-l-arginine ethyl ester as substrate. Papain isolated from this source appears to have the same catalytic-centre characteristics as papain isolated previously from latex produced by harsher methods. The catalysis of the hydrolysis of α-N-benzoyl-l-arginine ethyl ester by the mixture of thiol proteinases extracted from spray-dried latex by application of the method of Kimmel & Smith (1954) appears to obey Michaelis–Menten kinetics. The presence of the other enzymes results in an increase in the value of Km and a decrease in the catalytic-centre activity (kcat.) relative to the values for the catalysis by papain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines B. S., Brocklehurst K. A spectrophotometric method for the detection of contaminant chymopapains in preparations of papain. Selective modification of one type of thiol group in the chymopapains by a two-protonic-state reagent. Biochem J. 1978 Jul 1;173(1):345–347. doi: 10.1042/bj1730345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines B. S., Stuchbury T., Brocklehurst K. Preparation and characterization of enzymes from spray-dried papaya (Carica papaya) latex [proceedings]. Biochem Soc Trans. 1978;6(1):255–258. doi: 10.1042/bst0060255. [DOI] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography by thiol-disulfide interchange. Methods Enzymol. 1974;34:531–544. doi: 10.1016/s0076-6879(74)34069-4. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kierstan M. P. Propapain and its conversion to papain: a new type of zymogen activation mechanism involving intramolecular thiol-disulphide interchange. Nat New Biol. 1973 Apr 11;242(119):167–170. doi: 10.1038/newbio242167a0. [DOI] [PubMed] [Google Scholar]
- DIXON M., WEBB E. C. Enzyme fractionation by salting-out: a theoretical note. Adv Protein Chem. 1961;16:197–219. doi: 10.1016/s0065-3233(08)60030-3. [DOI] [PubMed] [Google Scholar]
- Flindt M. L. Respiratory hazards from papain. Lancet. 1978 Feb 25;1(8061):430–432. doi: 10.1016/s0140-6736(78)91216-3. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Intramolecular inhibition by enzyme of site-specific modification reactions can mask pKa values characteristic of the reaction pathway: do the side chains of aspartic acid-158 and lysine-156 of papain form an ion-pair? [proceedings]. Biochem Soc Trans. 1978;6(1):250–252. doi: 10.1042/bst0060250. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Preparation of fully active ficin from Ficus glabrata by covalent chromatography and characterization of its active centre by using 2,2'-depyridyl disulphide as a reactivity probe. Biochem J. 1976 Nov;159(2):221–234. doi: 10.1042/bj1590221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. W. Isolation and characterization of papaya peptidase A from commercial chymopapain. Biochemistry. 1975 Aug 12;14(16):3695–3700. doi: 10.1021/bi00687a028. [DOI] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole as a reactivity probe for the investigation of the thiol proteinases. evidence that ficin and bromelain may lack carboxyl groups conformationally equivalent to that of aspartic acid-158 of papain. Biochem J. 1976 Nov;159(2):235–244. doi: 10.1042/bj1590235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITAKER J. R., BENDER M. L. KINETICS OF PAPAIN-CATALYZED HYDROLYSIS OF ALPHA-N-BENZOYL-L-ARGININE ETHYL ESTER AND ALPHA-N-BENZOYL-L-ARGININAMIDE. J Am Chem Soc. 1965 Jun 20;87:2728–2737. doi: 10.1021/ja01090a034. [DOI] [PubMed] [Google Scholar]
- Wharton C. W., Cornish-Bowden A., Brocklehurst K., Crook E. M. Kinetics of the hydrolysis of N-benzoyl-L-serine methyl ester catalysed by bromelain and by papain. Analysis of modifier mechanisms by lattice nomography, computational methods of parameter evaluation for substrate-activated catalyses and consequences of postulated non-productive binding in bromelain- and papain-catalysed hydrolyses. Biochem J. 1974 Aug;141(2):365–381. doi: 10.1042/bj1410365. [DOI] [PMC free article] [PubMed] [Google Scholar]