Abstract
Aim:
According to the results of the MASTRO I living systematic review and meta-analysis, use of the EmboTrap Revascularization® Device in the treatment of acute ischemic stroke (AIS) results in higher rates of good functional outcomes (90-day modified Rankin Scale [mRS] 0–2) compared with use of the Trevo® Retriever or the Solitaire™ Revascularization Device. The aim of this analysis was to assess the potential economic impact of achieving improved functional outcomes for three commonly used stent retrievers (SRs) in the treatment of AIS.
Methods:
An economic model with short-term and long-term costs, representing a healthcare system perspective was developed using a decision tree to simulate a cohort of 1000 hypothetical patients treated for AIS with mechanical thrombectomy (MT) using EmboTrap, Trevo or Solitaire SRs. Based on the proportion of patients who achieved a 90-day mRS score of 0–2 or 3–5 for each device reported in MASTRO I (excluding patients not surviving after 90 days), this model estimated per-patient costs and the associated incremental cost savings. Results are reported from the healthcare system perspective in the US, Canada, the UK, Sweden, Germany, France, Italy, Spain, Belgium and The Netherlands.
Results:
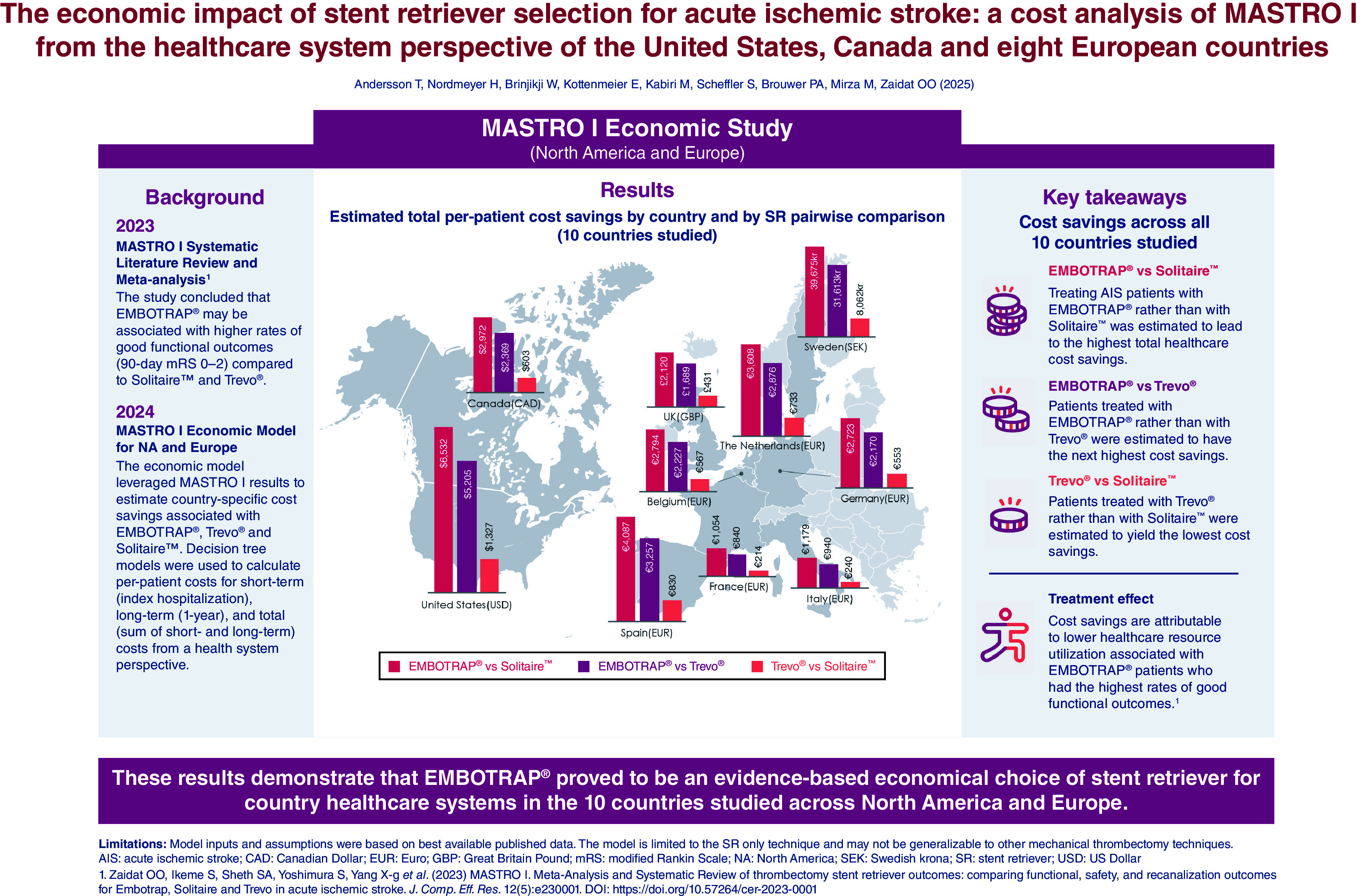
Across all ten countries, the use of EmboTrap during MT was associated with the lowest short-term (ranging from €8412 in Italy to $66,525 in the US), long-term (ranging from €5249 in Italy to $25,757 in the US) and total (ranging from €13,661 in Italy to $92,282 in the US) per-patient costs. The total per-patient cost was higher with Trevo (ranging from €14,601 in Italy to $97,487 in the US) and Solitaire (ranging from €14,840 in Italy to $98,814 in the US). Cost savings were highest when comparing EmboTrap versus Solitaire, followed by EmboTrap versus Trevo, with Trevo versus Solitaire having the smallest cost savings. Results of sensitivity and scenario analyses supported the robustness of the base-case results.
Conclusion:
Across the ten countries, treating patients with AIS with EmboTrap resulted in lower short-term, long-term and total costs to the payer. With rising healthcare costs and limited hospital budgets, these results suggest EmboTrap proves to be an evidence-based economical choice of SR for hospitals and healthcare systems.
Keywords: acute ischemic stroke, cost, economic, EmboTrap, MASTRO I, mechanical thrombectomy, modified Rankin Scale, Solitaire, stent retriever, Trevo
Plain language summary
What is this article about?
Mechanical thrombectomy (MT) is the current standard of care for the treatment of acute ischemic stroke and can be performed using stent retrievers (SRs), aspiration catheters or a combination of both. MASTRO I, a recently published systematic review and meta-analysis, compared outcomes following MT for three commonly used SRs (EmboTrap Revascularization® Device, Trevo® Retriever and Solitaire™ Revascularization Device) and found that the SR used during MT can impact functional outcomes. Data from MASTRO I reported that compared with Trevo and Solitaire, treatment with EmboTrap was associated with improved functional outcomes. As better functional outcomes may result in lower healthcare resource utilization, SR choice during MT may have downstream economic implications. Therefore, the aim of the current analysis was to compare the total (short- and long-term) economic outcomes with the use of EmboTrap, Trevo and Solitaire in the US, Canada, the UK, Sweden, Germany, France, Italy, Spain, Belgium and The Netherlands.
What were the results?
EmboTrap use was associated with the lowest short-term, long-term and total costs in all ten countries evaluated, followed by Trevo and Solitaire.
What do the results mean?
These results may assist physicians and hospital stakeholders in supporting evidence-based decision making when selecting an optimal choice of SR for MT. The results from this analysis suggest that, beyond device cost, optimizing patient functional outcomes through device selection can impact downstream economic consequences across all ten countries studied.
Graphical abstract
Stroke is a leading cause of death and disability worldwide [1,2,3], and survivors often experience long-term deficits, with approximately 90% experiencing disability [4]. As a result, patients, their caregivers [5,6] and healthcare systems [1] are substantially impacted by the disease.
Total direct medical costs related to stroke are projected to more than double by 2035 due to the global aging population particularly across high-income countries [1], which will significantly increase the already substantial economic burden of stroke. Care for patients who have experienced a stroke was estimated to cost the US healthcare system $56.2 billion USD in direct and indirect costs between 2019 and 2020, with the direct cost of stroke estimated to be $34.5 billion USD [7]. In Canada, the total stroke-related healthcare costs in the first year following stroke was estimated to be $2 billion Canadian dollars (CAD) in 2021 [8]. Across Europe, stroke-related healthcare costs were estimated to be €27 billion in 2017 [9]. Therefore, opportunities to reduce stroke-related costs should be prioritized for consideration.
Approximately 63–87% of all strokes are ischemic [1,3], with 31–46% caused by a large vessel occlusion (LVO) [10,11,12]. Mechanical thrombectomy (MT) is the current standard of care for eligible patients with acute ischemic stroke (AIS) due to LVO [13,14,15]. Compared with standard of care of intravenous thrombolysis alone, MT results in better clinical outcomes [13,16], including good functional outcomes defined by modified Rankin Scale (mRS) score of 0–2 at 90-days post-stroke [13,16], and is more cost-effective [17,18]. During MT, different techniques and devices, such as aspiration catheters, stent retrievers (SRs) or a combination of both, can be used to restore blood flow. The use of SRs is well established and recommended in clinical guidelines [13,14,15], however individual SRs possess unique design characteristics and mechanisms known to influence recanalization and functional outcomes during and following MT [19,20,21,22].
The results of a recent systematic literature review and meta-analysis, MASTRO I, suggest that SR choice may impact functional outcomes following MT with a SR used as the first-line technique [20]. The use of the EmboTrap Revascularization® Device during MT resulted in significantly higher rates of 90-day mRS 0–2 (57.4%) compared with the use of the Solitaire™ Revascularization Device (45.3%, p < 0.001) or the Trevo® Retriever (50.0%, p = 0.013) [20]. As post-stroke mRS score has been shown to directly correlate with medical costs, patients with 90-day mRS 3–5 (i.e., functionally dependent) are likely to have higher healthcare-related costs than patients with 90-day mRS 0–2 (i.e., functionally independent) [23,24].
Currently, no published research has estimated the economic impact of SR selection following MT for AIS. The aim of this analysis was to assess the economic impact of achieving improved functional outcomes with the use of three commonly used SRs for ten countries from the healthcare system perspective.
Methods
Study design
The cost model was designed to compare the cost of using EmboTrap, Trevo and Solitaire, based on their differential proportion of patients in mRS 0–2 versus mRS 3–5 at 90 days post MT. The model assessed one-year costs from the healthcare systems perspective for ten countries: the US, Canada, the UK, Sweden, Germany, France, Italy, Spain, Belgium and The Netherlands. Costs included index hospitalization costs as well as post-discharge healthcare costs up to 1-year post-stroke. The details of cost outcomes are presented in the following sections.
Model structure
Three decision trees were developed per country using R (version 3.6.1) for three pairwise comparisons: EmboTrap versus Trevo, EmboTrap versus Solitaire, and Trevo versus Solitaire. Each decision tree simulated a cohort of 1000 adult patients with AIS due to emergent large vessel occlusion treated with MT using either EmboTrap, Trevo or Solitaire as the first-line SR and included two health states: good functional outcomes (90-day mRS 0–2) or poor functional outcomes (90-day mRS 3–5) (Figure 1). Patient cohort represented the pooled patient characteristics, such as age, sex and recanalization outcomes, as reported in MASTRO I [20].
Figure 1. . Decision trees for stent retriever selection in treating acute ischemic stroke.
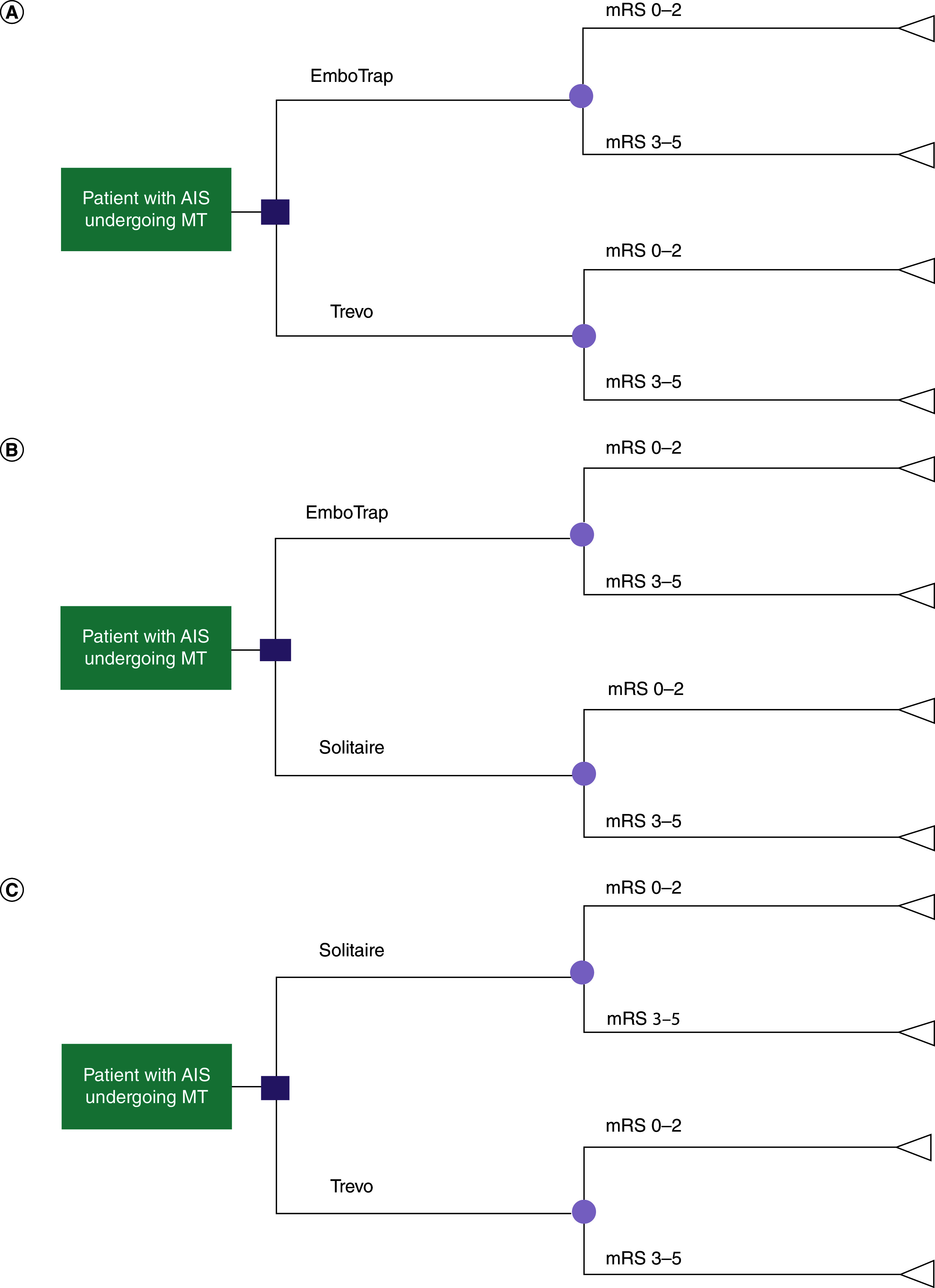
The decision tree depicts possible branches of treatments for the treatment of AIS for patients who were treated with (A) EmboTrap versus Trevo, (B) EmboTrap versus Solitaire, (C) Solitaire versus Trevo and were alive 90-days post-treatment. The square represents the node between receiving treatment with EmboTrap and either Solitaire or Trevo. The circle represents the node between the two possible mRS scores the patient can achieve: mRS 0–2 or 3–5. The triangle represents the economic outcomes associated with achieving the mRS score.
AIS: Acute ischemic stroke; mRS: modified Rankin Scale; MT: Mechanical thrombectomy; SR: Stent retriever.
Clinical efficacy & cost inputs
Clinical efficacy inputs included the proportion of patients who achieved good functional outcomes (90-day mRS 0–2) versus poor functional outcomes (90-day mRS 3–5) by device obtained from MASTRO I [20] (Table 1). We assumed all other clinical efficacy components related to SR devices were similar across patient cohorts.
Table 1. . Model inputs and probabilistic sensitivity analyses distributions.
| Input parameter |
Value |
Probability distribution and parameters |
Source (year) |
Ref. |
|
|---|---|---|---|---|---|
| Distribution of patients by mRS category‡ | |||||
| EmboTrap | |||||
| mRS 0–2 | 64.64% | Multinomial (0.574, 0.314, 0.112) | Zaidat et al. (2023) | [20] | |
| mRS 3–5 | 35.36% | ||||
| Trevo | |||||
| mRS 0–2 | 58.48% | Multinomial (0.500, 0.355, 0.145) | Zaidat et al. (2023) | [20] | |
| mRS 3–5 | 41.52% | ||||
| Solitaire | |||||
| mRS 0–2 | 56.91% | Multinomial (0.453, 0.343, 0.204) | Zaidat et al. (2023) | [20] | |
| mRS 3–5 | 43.09% | ||||
| Short-term index hospitalization inputs | |||||
| Mean index hospitalization length of stay (days) | |||||
| mRS 0–2 | 8.9 | Gamma† (α = 168.66, β = 19.052) | Zaidat et al. (2023) and Dewilde et al. (2017) | [20] and [24] | |
| mRS 3–5 | 18.4 | Gamma (α = 168.66, β = 9.240) | |||
| Index hospitalization cost per day (2023 values) by country | |||||
| US (USD) | $5433 | Gamma† (α = 168.66, β = 0.0312) | Simpson et al. (2017) | [25] | |
| Canada (CAD) | $1315 | Gamma† (α = 168.66, β = 0.1290) | Mittmann et al. (2012) | [26] | |
| UK (GBP) | £766 | Gamma† (α = 168.65, β = 0.2214) | Luengo-Fernandez et al. (2020) | [9] | |
| Sweden (SEK) | 11,578kr | Gamma† (α = 168.66, β = 0.1656) | Luengo-Fernandez et al. (2020) | [9] | |
| Germany (EUR) | €950 | Gamma† (α = 168.69, β = 0.1786) | Luengo-Fernandez et al. (2020) | [9] | |
| France (EUR) | €690 | Gamma† (α = 168.65, β = 0.2458) | Luengo-Fernandez et al. (2020) | [9] | |
| Italy (EUR) | €687 | Gamma† (α = 168.65, β = 0.2469) | Luengo-Fernandez et al. (2020) | [9] | |
| Spain (EUR) | €758 | Gamma† (α = 168.66, β = 0.2237) | Luengo-Fernandez et al. (2020) | [9] | |
| Belgium (EUR) | €1028 | Gamma† (α = 168.65, β = 0.1650) | Luengo-Fernandez et al. (2020) | [9] | |
| The Netherlands (EUR) | €1674 | Gamma† (α = 168.66, β = 0.1013) | Luengo-Fernandez et al. (2020) | [9] | |
| Long-term 1-year post-index hospitalization inputs | |||||
| 1-year post-stroke care costs (2023 values) by country | |||||
| US (USD) | mRS 0–2 | $14,038 | Gamma† (α = 168.67, β = 0.0120) | Shireman et al. (2017) | [18] |
| mRS 3–5 | $47,180 | Gamma† (α = 168.68, β = 0.0036) | |||
| Canada (CAD) | mRS 0–2 | $19,506 | Gamma† (α = 168.68, β = 0.0087) | Mittmann et al. (2012) | [26] |
| mRS 3–5 | $45,525 | Gamma† (α = 168.68, β = 0.0037) | |||
| UK (GBP) | mRS 0–2 | £4452 | Gamma† (α = 168.66, β = 0.0381) | Lobotesis et al. (2016) | [27] |
| mRS 3–5 | £24,632 | Gamma† (α = 168.68, β = 0.0069) | |||
| Sweden (SEK) | mRS 0–2 | 62,208kr | Gamma† (α = 168.66, β = 0.0308) | Lekander et al. (2017) | [32] |
| mRS 3–5 | 466,020kr | Gamma† (α = 168.69, β = 0.0041) | |||
| Germany (EUR) | mRS 0–2 | €2049 | Gamma† (α = 168.66, β = 0.0828) | Oliveira Gonçalves et al. (2023) | [28] |
| mRS 3–5 | €28,297 | Gamma† (α = 168.69, β = 0.0060) | |||
| France (EUR) | mRS 0–2 | €7901 | Gamma† (α = 168.66, β = 0.0215) | Barral et al. (2020) | [29] |
| mRS 3–5 | €15,013 | Gamma† (α = 168.67, β = 0.0113) | |||
| Italy (EUR) | mRS 0–2 | €2150 | Gamma† (α = 168.65, β = 0.0789) | Fattore et al. (2012) | [30] |
| mRS 3–5 | €10,913 | Gamma† (α = 168.67, β = 0.0116) | |||
| Spain (EUR) | mRS 0–2 | €3177 | Gamma† (α = 168.65, β = 0.0534) | de Andrés-Nogales et al. (2017) | [31] |
| mRS 3–5 | €48,887 | Gamma† (α = 168.68, β = 0.0347) | |||
| Belgium (EUR) | mRS 0–2 | €4510 | Gamma† (α = 168.65, β = 0.0458) | Dewilde et al. (2017) | [24] |
| mRS 3–5 | €30,937 | Gamma† (α = 168.68, β = 0.0067) | |||
| The Netherlands (EUR) | mRS 0–2 | €7067 | Gamma† (α = 168.66, β = 0.0240) | Pinckaers et al. (2024) | [33] |
| mRS 3–5 | €37,923 | Gamma† (α = 168.69, β = 0.0045) | |||
α and β present shape and rate parameters for inputs following a Gamma distribution and are rounded to two and four decimal points, respectively.
Data reweighted to exclude mRS 6.
CAD: Canadian dollar; EUR: Euro; GBP: Great Britain Pound; mRS: 90-day modified Rankin Scale score; NA: Not applicable; NR: not reported; SEK: Swedish krona; USD: United States dollar.
This analysis included costs obtained from published literature. Cost inputs did not differ by choice of SR device, leaving clinical efficacy inputs as the driver of model outcomes.
Short-term cost inputs were calculated for mRS 0–2 and 3–5 health states by multiplying the weighted average length of stay (LOS) by the per-patient cost for hospital day following stroke [9,25,26] for each state. We calculated the weighted average LOS for mRS 0–2 and 3–5 health states by aggregating the distribution of patient volume across mRS 0–5 categories and their respective average LOS reported in the literature [20,24]. Device cost was not included in the model.
Weighted average long-term cost inputs for mRS 0–2 and 3–5 states were calculated using the per-patient long-term costs by 90-day mRS score (0–5) [18,24,26–33], aggregated using the distribution of patients in 90-day mRS score (0–5) [20]. Per-patient costs by 90-day mRS score were obtained from published literature (Table 1).
Costs captured in the long-term cost inputs included direct long-term care costs and social care (direct other) costs, the latter of which reflected nursing or professional care, home care, institutional or residential care, and/or special housing costs. Where possible, indirect costs were removed from the long-term cost inputs. Figure 2 outlines the long-term cost categories reported for each country by source. Due to limitations in long-term data reported for Canada, Germany, France and The Netherlands, additional assumptions were required to impute direct long-term care costs (Supplementary Methods).
Figure 2. . Comparison of country-specific long-term costs included in the model.
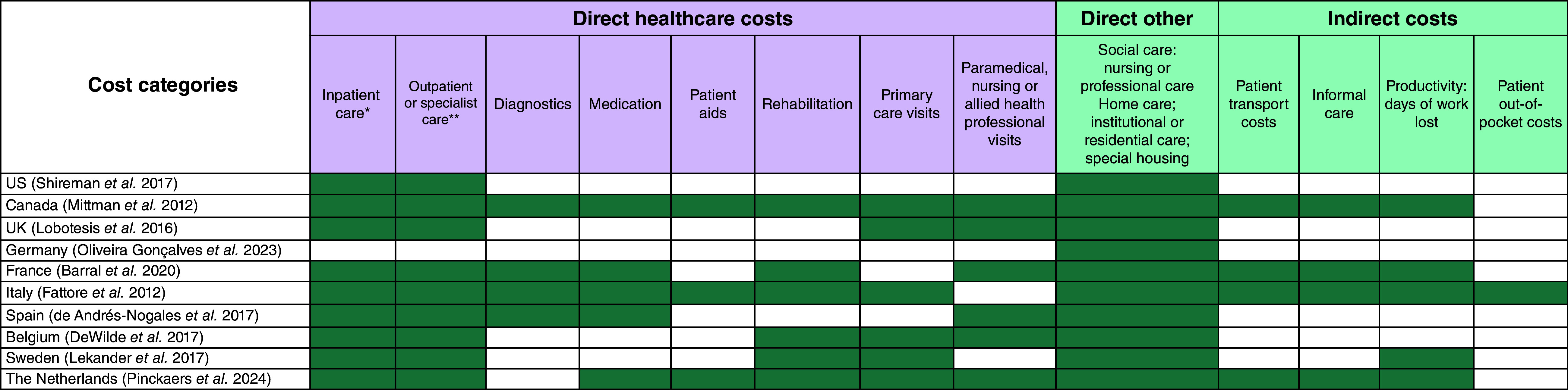
The figure depicts the cost categories that are included in the country-specific long-term costs included in the model. Green cells indicate that the cost is included in the long-term cost input used in the model.
*Includes visits to emergency room.
**Also includes day cases.
Costs for the US, Canada, European countries and the UK were included in the model in local currency (i.e., USD, CAD, Euros and Great Britain Pounds [GBP], respectively). Belgian costs were sourced from a publication that reported costs in USD [24], thus costs were converted to Euros [34]. Costs for Sweden were reported in Euros from the source publication [32]. Unless otherwise noted, costs for all countries were inflated to 2023 values using the health-specific Consumer Price Indices from the International Monetary Fund [35]. The cost inputs in the model were assumed to be the same regardless of the SR device used (Table 1).
Model outcomes
The cost model estimated per-patient cost savings (incremental costs) for each country based on the pairwise device comparisons of total costs, comprised of short- and long-term costs, described below. In addition to per-patient incremental cost outcomes, the percent differences in total cost for each pairwise comparison were calculated to provide a normalized assessment of cost savings. Cost outcomes were reported in the same currency they were input into the model, with the exception of Sweden where results are reported in local currency (2023 Swedish Krona [SEK]).
Short-term costs included the costs accrued during index hospitalization after the MT procedure. Per-patient short-term costs were calculated using the average per-patient cost of mRS 0–2 and 3–5 states for hospitalization LOS multiplied by their respective proportion of patients in each state for each device. Long-term costs included the healthcare costs accrued from the time of patient discharge to one-year post-stroke. Per-patient long-term costs were calculated using the average per-patient cost of mRS 0–2 and 3–5 states for 1 year post MT weighted by the device-specific proportion of patients in each state. Figure 3 depicts the overall time horizon for the cost model.
Figure 3. . Overall time horizon of the short- and long-term analyses.
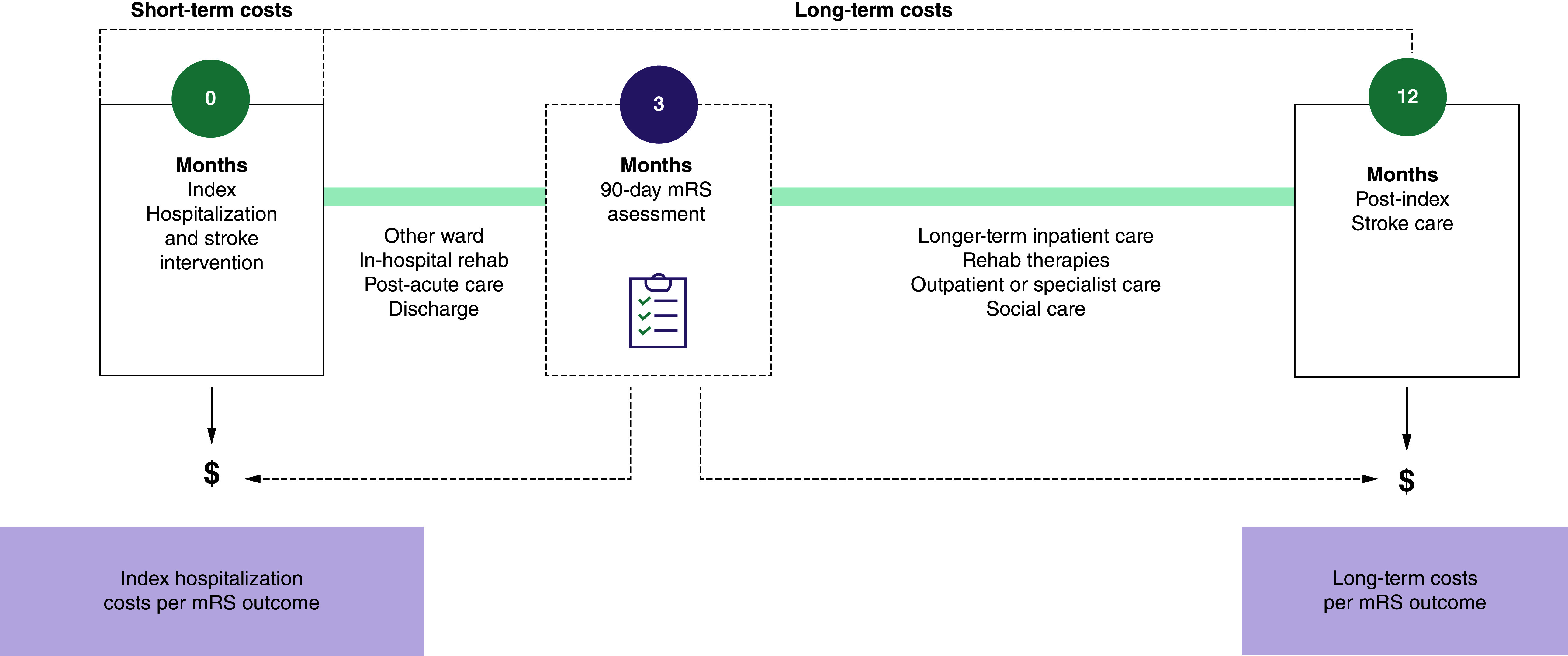
mRS: 90-day modified Rankin Scale score.
As cost estimates for death (mRS 6) were not consistently reported in the source publications, this analysis focused on patients who survived to 90 days. Device-specific patient volume for mRS 0–2 and 3-5 were proportionally adjusted to exclude mRS 6 and preserve differences in treatment effect (Table 1).
Sensitivity & scenario analyses
Probabilistic sensitivity analyses were conducted based on 10,000 iterations of parametric Monte Carlo simulations. Input parameters varied in each iteration of the probabilistic sensitivity analyses according to the probability distributions reported in Table 1. 95% credible intervals (CrIs) were estimated for all model outcomes. Due to the hypothetical patient population used in this model, Crls are used to represent the range between which 95% of probabilistic sensitivity analysis results fall.
Deterministic one-way sensitivity analyses were performed for each country by varying individual input parameters by ± 15%. The proportional distribution of patients across mRS categories was conserved to preserve logic and ensure patient percentages always summed to 100%.
A probabilistic scenario analysis was conducted based on the statistical outlier analysis performed in MASTRO I [20], which excluded the studies that reported values that differed significantly from the overall effect in the meta-analysis. In this scenario analysis, the proportion of patients that achieved mRS 0–2 at 90 days was reduced for EmboTrap (from 57.4 to 54.9%), increased for Solitaire (from 45.3 to 46.2%) and did not change for Trevo (50.0%) [20].
Results
Deterministic base-case analyses
Across all ten countries, patients treated with EmboTrap were estimated to have the lowest short-term costs, long-term costs and total costs. Costs were higher in the cohort treated with Trevo, and highest in the cohort treated with Solitaire (Table 2). The estimated cost savings over the one-year period were greatest for EmboTrap versus Solitaire, followed by EmboTrap versus Trevo, with the smallest cost savings reported between Trevo versus Solitaire.
Table 2. . Deterministic base-case results for EmboTrap, Trevo and Solitaire.
| Country and device | Short-term cost | Long-term cost | Total cost | Per-patient incremental cost compared with EmboTrap | Per-patient incremental cost compared with Trevo |
|---|---|---|---|---|---|
| US (USD) | |||||
| EmboTrap | $66,525 | $25,757 | $92,282 | – | – |
| Trevo | $69,689 | $27,799 | $97,487 | $5205 | – |
| Solitaire | $70,495 | $28,319 | $98,814 | $6532 | $1327 |
| Canada (CAD) | |||||
| EmboTrap | $16,102 | $28,707 | $44,808 | – | – |
| Trevo | $16,868 | $30,309 | $47,177 | $2369 | – |
| Solitaire | $17,063 | $30,718 | $47,780 | $2972 | $603 |
| UK (GBP) | |||||
| EmboTrap | £9380 | £11,588 | £20,967 | – | |
| Trevo | £9826 | £12,830 | £22,656 | £1689 | – |
| Solitaire | £9940 | £13,147 | £23,087 | £2120 | £431 |
| Sweden (SEK) | |||||
| EmboTrap | 141,761kr | 204,998kr | 346,770kr | – | – |
| Trevo | 148,511kr | 229,884kr | 378,383kr | 31,613kr | – |
| Solitaire | 138,923kr | 236,215kr | 386,445kr | 39,675kr | 8062kr |
| Germany (EUR) | |||||
| EmboTrap | €11,632 | €11,330 | €22,963 | – | – |
| Trevo | €12,186 | €12,947 | €25,133 | €2170 | – |
| Solitaire | €12,327 | €13,359 | €25,686 | €2723 | €553 |
| France (EUR) | |||||
| EmboTrap | €8448 | €10,416 | €18,865 | – | – |
| Trevo | €8851 | €10,853 | €19,705 | €840 | – |
| Solitaire | €8953 | €10,965 | €19,919 | €1054 | €214 |
| Italy (EUR) | |||||
| EmboTrap | €8412 | €5249 | €13,661 | – | – |
| Trevo | €8812 | €5788 | €14,601 | €940 | – |
| Solitaire | €8914 | €5927 | €14,840 | €1179 | €240 |
| Spain (EUR) | |||||
| EmboTrap | €9282 | €19,340 | €28,621 | – | – |
| Trevo | €9723 | €22,156 | €31,879 | €3257 | – |
| Solitaire | €9835 | €22,874 | €32,709 | €4087 | €830 |
| Belgium (EUR) | |||||
| EmboTrap | €12,587 | €13,854 | €26,442 | – | – |
| Trevo | €13,187 | €15,482 | €28,668 | €2227 | – |
| Solitaire | €13,339 | €15,897 | €29,236 | €2794 | €567 |
| The Netherlands (EUR) | |||||
| EmboTrap | €20,497 | €17,979 | €38,476 | – | – |
| Trevo | €21,473 | €19,879 | €41,351 | €2876 | – |
| Solitaire | €21,721 | €20,363 | €42,084 | €3608 | €733 |
Results for Germany represent both 2023 and 2024 values due to the use of 2024 physician fees to calculate long-term costs.
CAD: Canadian dollar; EUR: Euro; GBP: Great Britain Pound; SEK: Swedish krona; USD: United States dollar.
As outlined in Table 2, compared with Trevo, EmboTrap was estimated to have per-patient incremental total cost savings of USD $5205 in the US, CAD $2369 in Canada, £1689 in the UK, SEK 31,613kr in Sweden, €2170 in Germany, €840 in France, €940 in Italy, €3257 in Spain, €2227 in Belgium and €2876 in The Netherlands.
Compared with Solitaire, EmboTrap was estimated to have per-patient incremental total cost savings of USD $6532 in the US, CAD $2972 in Canada, £2120 in the UK, SEK 39,675kr in Sweden, €2723 in Germany, €1054 in France, €1179 in Italy, €4087 in Spain, €2794 in Belgium and €3608 in The Netherlands (Table 2).
Relative to Solitaire, Trevo was estimated to have per-patient incremental total cost savings of USD $1327 in the US, CAD $603 in Canada, £431 in the UK, SEK 8062kr in Sweden, €553 in Germany, €214 in France, €240 in Italy, €830 in Spain, €567 in Belgium and €733 in The Netherlands (Table 2).
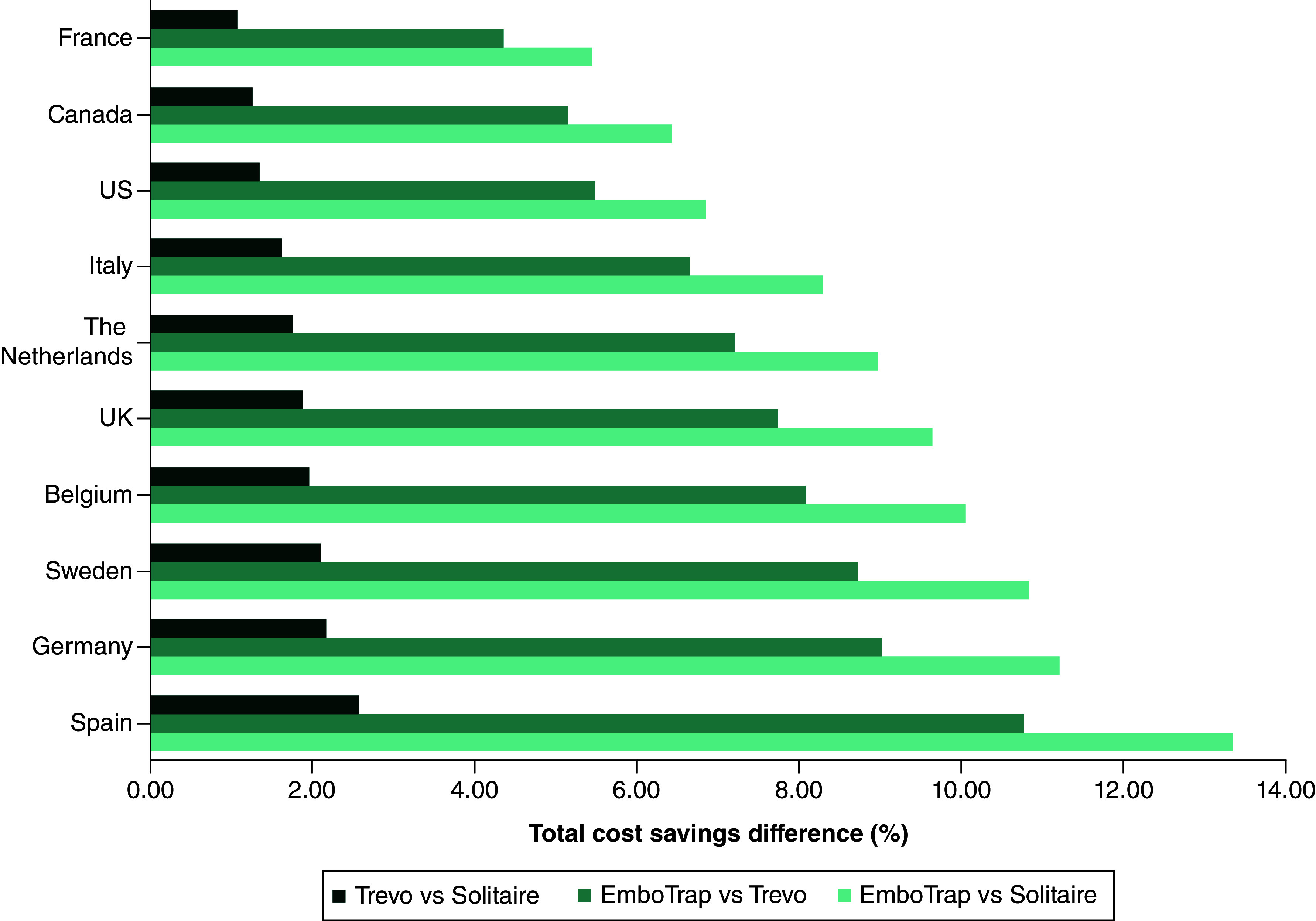
The normalized assessment of total cost, demonstrated by the percent difference in total costs, indicates that the greatest magnitude of cost savings for all pairwise comparisons was estimated for Spain and Germany, with France having the lowest cost savings (Figure 4).
Figure 4. . Per-patient pairwise comparison of total cost savings.
Probabilistic sensitivity analyses
The probabilistic sensitivity analyses aligned with the deterministic base-case results, with EmboTrap yielding the lowest short-term, long-term and total costs. Trevo, followed by Solitaire, had higher costs. Like the base-case, cost savings were the highest for EmboTrap versus Solitaire, followed by EmboTrap versus Trevo, with Trevo versus Solitaire yielding the lowest cost savings (Table 3).
Table 3. . Economic outcomes of probabilistic sensitivity analyses for EmboTrap, Trevo and Solitaire.
| Country and device | Short-term cost Mean (95% CrI) |
Long-term cost Mean (95% CrI) |
Total cost Mean (95% CrI) |
Per-patient incremental cost compared with EmboTrap Mean (95% CrI) |
Iterations with cost savings using EmboTrap | Per-patient incremental cost compared with Trevo Mean (95% CrI) |
Iterations with cost savings using Trevo |
|---|---|---|---|---|---|---|---|
| US (USD) | |||||||
| EmboTrap | $65,797 (65,674; 65,920) |
$25,637 (25,607; 25,668) |
$91,434 (91,306; 91,562) |
– | – | – | – |
| Trevo | $68,925 (68,795; 69,054) |
$27,673 (27,639; 27,706) |
$96,597 (96,463; 96,732) |
$5163 (4580; 5747) |
99.62% | – | – |
| Solitaire | $69,729 (69,598; 69,861) |
$28,199 (28,164; 28,233) |
$97,928 (97,790; 98,066) |
$6494 (5900; 7088) |
99.92% | $1331 (722; 1939) |
74.92% |
| Canada (CAD) | |||||||
| EmboTrap | $15,925 (15,896; 15,955) |
$28,576 (28,545; 28,608) |
$44,502 (44,458; 44,546) |
– | – | – | – |
| Trevo | $16,682 (16,651; 16,714) |
$30,174 (30,140; 30,208) |
$46,856 (46,810; 46,903) |
$2354 (2153; 2557) |
99.62% | – | – |
| Solitaire | $16,877 (16,845; 16,909) |
$30,587 (30,552; 30,622) |
$47,464 (47,416; 47,513) |
$2962 (2756; 3169) |
99.92% | $608 (395; 820) |
74.92% |
| UK (GBP) | |||||||
| EmboTrap | £9277 (9259; 9294) |
£11,532 (11,517; 11,547) |
£20,809 (20,785; 20,832) |
– | – | – | – |
| Trevo | £9718 (9699; 9736) |
£12,772 (12,755; 12,789) |
£22,489 (22,464; 22,515) |
£1680 (1571; 1790) |
99.62% | – | – |
| Solitaire | £9831 (9813; 9850) |
£13,092 (13,074; 13,110) |
£22,923 (22,896; 22,949) |
£2114 (2002; 2226) |
99.92% | £434 (317; 550) |
74.92% |
| Sweden (SEK) | |||||||
| EmboTrap | 140,212kr (139,952; 140,472) |
204,003kr (203,721; 204,286) |
344,215kr (343,819; 344,622) |
– | – | – | – |
| Trevo | 146,883kr (146,600; 147,154) |
228,810kr (228,482; 229,126) |
375,681kr (375,251; 376,122) |
31,466kr (29,623; 33,309) |
99.62% | – | – |
| Solitaire | 148,590kr (148,319; 148,873) |
235,209kr (234,870; 235,548) |
383,810kr (383,358; 384,263) |
39,584kr (37,685; 41,484) |
99.92% | 8118kr (6139; 10,097) |
74.92% |
| Germany (EUR) | |||||||
| EmboTrap | €11,505 (11,484; 11,527) |
€11,274 (11,257; 11,292) |
€22,779 (22,751; 22,808) |
– | – | – | – |
| Trevo | €12,052 (12,029; 12,075) |
€12,886 (12,867; 12,906) |
€24,938 (24,908; 24,969) |
€2159 (2028; 2290) |
99.62% | – | – |
| Solitaire | €12,193 (12,170; 12,216) |
€13,303 (13,282; 13,323) |
€25,495 (25,463; 25,527) |
€2716 (2581; 2851) |
99.92% | €557 (417; 697) |
74.92% |
| France (EUR) | |||||||
| EmboTrap | €8356 (8341; 8372) |
€10,369 (10,358; 10,381) |
€18,726 (18,706; 18,745) |
– | – | – | – |
| Trevo | €8754 (8737; 8770) |
€10,806 (10,794; 10,818) |
€19,560 (19,539; 19,580) |
€834 (745; 923) |
99.62% | – | – |
| Solitaire | €8856 (8839; 8872) |
€10,919 (10,907; 10,931) |
€19,775 (19,754; 19,796) |
€1049 (959; 1139) |
99.92% | €215 (123; 307) |
74.92% |
| Italy (EUR) | |||||||
| EmboTrap | €8320 (8304; 8336) |
€5224 (5217; 5231) |
€13,544 (13,527; 13,561) |
– | – | – | – |
| Trevo | €8715 (8699; 8732) |
€5762 (5754; 5770) |
€14,477 (14,459; 14,496) |
€934 (855; 1013) |
99.62% | – | – |
| Solitaire | €8817 (8801; 8834) |
€5901 (5893; 5909) |
€14,718 (14,699; 14,737) |
€1174 (1094; 1255) |
99.92% | €241 (158; 324) |
74.92% |
| Spain (EUR) | |||||||
| EmboTrap | €9167 (9150; 9184) |
€19,226 (19,196; 19,256) |
€28,393 (28,358; 28,428) |
– | – | – | – |
| Trevo | €9603 (9585; 9621) |
€22,025 (21,991; 22,060) |
€31,628 (31,589; 31,668) |
€3235 (3068; 3402) |
99.59% | – | – |
| Solitaire | €9714 (9696; 9733) |
€22,742 (22,707; 22,778) |
€32,456 (32,416; 32,497) |
€4063 (3893; 4234) |
99.92% | €828 (648; 1009) |
73.98% |
| Belgium (EUR) | |||||||
| EmboTrap | €12,450 (12,426; 12,473) |
€13,788 (13,769; 13,806) |
€26,237 (26,206; 26,268) |
– | – | – | – |
| Trevo | €13,042 (13,017; 13,066) |
€15,411 (15,389; 15,432) |
€28,452 (28,419; 28,485) |
€2215 (2073; 2357) |
99.62% | – | – |
| Solitaire | €13,194 (13,169; 13,219) |
€15,830 (15,807; 15,852) |
€29,024 (28,989; 29,058) |
€2786 (2640; 2932) |
99.92% | €571 (420; 723) |
74.92% |
| The Netherlands (EUR) | |||||||
| EmboTrap | €20,267 (20,230; 20,305) |
€17,879 (17,856; 17,902) |
€38,146 (38,102; 38,191) |
– | – | – | – |
| Trevo | €21,235 (21,196; 21,274) |
€19,775 (19,749; 19,801) |
€41,010 (40,962; 41,058) |
€2864 (2658; 3070) |
99.64% | – | – |
| Solitaire | €21,478 (21,438; 21,517) |
€20,251 (20,224; 20,278) |
€41,729 (41,680; 41,778) |
€3583 (3373; 3793) |
99.90% | €719 (502; 936) |
73.67% |
Probabilistic analyses were based on parametric Monte Carlo simulations with 10,000 iterations.
Results for Germany represent both 2023 and 2024 values due to the use of 2024 physician fees to calculate long-term costs.
CAD: Canadian dollar; CrI: Credible interval; EUR: Euro; GBP: Great Britain Pound; SEK: Swedish krona; USD: United States dollar.
As outlined in Table 3, compared with Trevo, EmboTrap was estimated to have per-patient incremental total cost savings of USD $5163 (95% Crl: 4580; 5747) in the US, CAD $2354 (95% Crl: 2153; 2557) in Canada, £1680 (95% Crl: 1571; 1790) in the UK, SEK 31,466 (95% Crl: 29,623; 33,039) in Sweden, €2159 (95% Crl: 2028; 2290) in Germany, €834 (95% Crl: 745; 923) in France, €934 (95% Crl: 855; 1013) in Italy, €3235 (95% Crl: 3068; 3402) in Spain, €2215 (95% Crl: 2073; 2357) in Belgium and €2864 (95% Crl: 2658; 3070) in The Netherlands.
Compared with Solitaire, EmboTrap was estimated to have per-patient incremental total cost savings of USD $6494 (95% Crl: 5900; 7088) in the US, CAD $2962 (95% Crl: 2756; 3169) in Canada, £2114 (95% Crl: 2002; 2226) in the UK, 39,584kr (95% Crl: 37,685; 41,484) in Sweden, €2716 (95% Crl: 2581; 2851) in Germany, €1049 (95% Crl: 959; 1139) in France, €1174 (95% Crl: 1094; 1255) in Italy, €4063 (95% Crl: 3893; 4234) in Spain, €2786 (95% Crl: 2640; 2932) in Belgium and €3583 (95% Crl: 3373; 3793) in The Netherlands (Table 3).
Relative to Solitaire, Trevo was estimated to have per-patient incremental total cost savings of USD $1331 (95% Crl: 722; 1939) in the US, CAD $608 (95% Crl: 395; 820) in Canada, £434 (95% Crl: 317; 550) in the UK, 8118kr (95% Crl: 6139; 10,097) in Sweden, €557 (95% Crl 417; 697) in Germany, €215 (95% Crl 123; 307) in France, €241 (95% Crl: 158; 324) in Italy, €828 (95% Crl: 648; 1009) in Spain, €571 (95% Crl: 420; 723) in Belgium and €719 (95% Crl: 502; 936) in The Netherlands (Table 3).
Across all ten countries, over 99% of iterations found the use of EmboTrap relative to Trevo or Solitaire resulted in per-patient cost savings. The use of Trevo relative to Solitaire was cost saving in over 73% of iterations in all countries (Table 3).
One-way sensitivity analyses
Across the three pairwise comparisons (EmboTrap versus Solitaire, EmboTrap versus Trevo, Solitaire versus Trevo) in the ten countries assessed, the one-way sensitivity analyses indicated that the results were the most sensitive to variations in the proportion of patients who achieved mRS 0–2 versus mRS 3–5 at 90 days (Supplementary Figures 1–10). Across all countries, the model was less sensitive to variations in the short-term hospitalization LOS, cost of stroke hospital day and 1-year costs for mRS 0–2 and 3–5.
Scenario analyses
Similar to the probabilistic sensitivity analyses results, the probabilistic scenario analyses estimated that the use of EmboTrap led to the lowest costs, followed by Trevo, then Solitaire. Across all ten countries, the highest per-patient cost savings were observed with the use of EmboTrap relative to Solitaire, followed by the use of EmboTrap relative to Trevo. The use of Trevo relative to Solitaire was associated with the lowest per-patient cost savings. The reported cost savings were reduced relative to the cost savings observed in the deterministic base-case and the probabilistic sensitivity analyses. Furthermore, fewer iterations were associated with costs savings (Supplementary Table 1).
Discussion
This study assessed the economic impact of improved functional outcomes associated with SR device selection during MT based on MASTRO I [20]. The costs were analyzed from the healthcare system perspective for the US, Canada, the UK, Sweden, Germany, France, Italy, Spain, Belgium and The Netherlands, representing the major Western countries where the three devices are marketed. The results suggest that improved functional outcomes achieved with EmboTrap may translate to lower per-patient total costs and, therefore, cost savings compared with the use of Trevo or Solitaire across all countries studied. The US, followed by Spain, had the highest absolute cost savings for each SR pairwise comparison, whereas France had the lowest. However, when assessing the normalized values of total cost, as demonstrated through percent difference in per-patient total cost, the greatest magnitude of cost savings was observed for Spain, followed by Germany, with France having the lowest magnitude. These results differ from those obtained when comparing the direct absolute values of cost savings, underscoring the nuanced dynamics of cost differentials across health systems and the potential caveats in comparing absolute costs across the countries.
The results of this analysis highlight the potential impact of SR device selection on the economic burden of stroke. Our study also shows that using a SR device with better functional outcomes produced greater cost savings in the long-term versus the short-term in five of the ten countries, highlighting the impact of decision making in an acute care setting, not only on patient outcomes, but also on healthcare resource utilization and costs in the long-term. Our results demonstrate that the economic burden of stroke is not simply a one-time expense associated with acute hospitalization; rather, the economic burden commences with index hospitalization and continues to accumulate over time. Our results also align with Johnson et al., Patel et al. and Lucas-Noll et al. which highlight the impact of acute care on long-term versus short-term care costs for stroke patients [36–38].
The results of this study also highlight the importance of considering differences between healthcare systems, treatment patterns and costs across various countries. The high total costs estimated for the US are not surprising given that medical costs in the US are among the highest in the world [39]. Resulting total costs in the US were driven by short-term costs, which were roughly 2.5x higher than estimated long-term costs across all three SRs. Germany, Italy, Belgium and The Netherlands also yielded short-term cost results that were higher than long-term costs, but to a lesser magnitude than the US (Table 2). Altogether, these country-specific differences in short- and long-term costs demonstrate that although EmboTrap was associated with cost savings in all countries of interest, the factors driving cost savings vary by country.
MASTRO I found that EmboTrap was associated with improved functional outcomes and reduced mortality at 90 days [20]. Further, per DeWilde et al. [24], hospital LOS can correlate with functional outcomes, with patients who achieve 90-day mRS 0–2 having a shorter LOS (8.9 days) compared with patients who achieve mRS 3–5 (18.4 days). This difference in LOS may translate to device-specific differences in healthcare resource use.
The results of this analysis provide valuable insights on SR selection for providers and healthcare policy decision-makers aiming to alleviate the clinical and economic burden of stroke. Though these results should be considered in conjunction with SR prices at the local payer level within each country. Given the healthcare market, disease burden and SR prices, exploring the country-specific cost-effectiveness for SR selection should also be considered. Cost-effectiveness analysis will better assess the long-term value for money given the impact of SR intervention on patients' health and each market's willingness to pay.
Our study has some limitations. First, although best-available costing data were leveraged per country, some assumptions were required to derive costing inputs used in the model. Device prices were not included due to lack of reliable and citable sources for country-specific and product-specific device price, variability of device prices over time and potential variability of prices within each country across different regions. Short-term and long-term cost data were sourced based on country specific calculations, different references, and represent unique healthcare structures. The analysis did not account for cost differences and performance influences associated with the use of rescue devices, aspiration catheters in combination techniques or BGCs. Additionally, the model did not specifically include other stroke-related health issues, such as recurrent stroke, as independent model health states. This is due to the model inputs being based on MASTRO I for mRS score and country-specific studies for cost estimates, in which many did not report this level of detail. Variables such as clot composition, operator skill, hospital volume and accessibility to stroke centers could not be adjusted for; these variables may influence both the clinical and economic outcomes associated with SR use. To derive long-term direct healthcare costs for Germany, France, Canada and The Netherlands, additional manipulations were required (Supplementary Methods). Furthermore, with publication years ranging from 2012 (Italy and Canada) to 2024 (The Netherlands), select sources used to estimate costs may be outdated, may not reflect current clinical practice and/or resource use due to the rapidly evolving stroke treatment landscape, and may not have increased in line with inflation over time. This limitation is underscored by the use of Belgian-specific LOS data from the Dewilde et al. study [24]. Together, this highlights the need for ongoing assessment of stroke-related cost data globally, not only to accurately reflect the current stroke-specific burden to healthcare systems but also to improve the precision of economic modeling. As updated cost data for stroke becomes available, the relationship between SR-related functional outcomes and the subsequent economic impact should be reassessed.
The second limitation is that the treatment effect in this model is determined based on reported 90-day mRS score associated with SR selection [20]. The mRS scores were grouped into ‘good’ and ‘poor’ functional outcomes (i.e., mRS 0–2 and 3–5, respectively) and may lack sensitivity in differences at the categorical level (mRS 0–5). Patients with mRS 6 were excluded because quality of life was not considered in this model and cost estimates were not reported for cases resulting in 90-day mortality. Further, mRS is a subjective disability scale commonly used to assess patient outcomes following stroke [13,40] which has inherent strengths and limitations when used to measure the well-being of individuals who have suffered a stroke. While some studies show variability in 90-day mRS, others have demonstrated the validity of mRS [41,42,43]. Studies show 43–76% of patients have unchanged 90-day mRS scores at 12 months [44,45,46,47], indicating that the 90-day mRS score may be a reliable measure of short- and long-term disability. In relation to functional independence, mRS score stability has been demonstrated with the proportion of patients achieving mRS 0–2 at 90-days remaining unchanged for ∼96% of these patients at 12 months [45], thereby supporting the use of 90-day mRS scores in predicting long-term clinical and economic outcomes.
Finally, the difference in economic outcomes demonstrated between the commonly used SRs evaluated (EmboTrap, Trevo and Solitaire) in the current model may be influenced by the geometric configuration of the SR [19]. Studies have demonstrated differences in recanalization outcomes between SRs, which may impact patient functional outcomes following MT [20,21,22]. EmboTrap is a third-generation dual-layer nitinol SR with a closed cell inner channel with a high radial force that is surrounded by an open-cell configuration for instant recanalization and a closed-cell configuration on the distal tip to catch clot fragments [19]. Trevo and Solitaire are both second-generation nitinol stents with a closed cell, barrel, peak-to-peak geometric configuration designed to leverage radial force alone to capture and remove the clot [19]. The difference in device design between EmboTrap, Trevo and Solitaire may influence the observed improvements in functional and, subsequently, economic outcomes. Data was not available to evaluate differences in clinical and economic outcomes for other third-generation SRs, such as Tigertriever (Rapid Medical), 3D Revascularization Device (Penumbra) and Eric (Microvention) [19].
Overall, this study sought to assess the relationship between SR-related functional outcomes and their subsequent economic impact following MT, and assumed all other procedural techniques were similar across cohorts. The results of MASTRO I suggest that SR choice may impact post-stroke patient functional outcomes, as demonstrated by EmboTrap's association with higher rates of functional independence followed by Trevo and then Solitaire [20]. The current study demonstrates that improved functional outcomes may be associated with lower healthcare costs and therefore cost savings, thereby reducing the clinical and economic burden of stroke across countries in Europe and North America. Overall, the results of the analysis support the importance of SR selection during MT to optimize clinical and economic outcomes in the short and long-term across healthcare systems.
Conclusion
Across the ten countries studied in this model, patients with AIS treated with EmboTrap alone on the first attempt were estimated to have the lowest healthcare costs, followed by those treated with Trevo, with those treated with Solitaire having the highest costs among patients who survived to 90 days. Based on total cost, the greatest cost savings over the 1-year study period were reported for EmboTrap versus Solitaire, followed by EmboTrap versus Trevo, with the smallest cost savings reported between Trevo versus Solitaire. The cost savings reported in this analysis are attributable to the lower healthcare resource utilization for patients treated with EmboTrap who had the highest rates of good functional outcomes (mRS 0–2). With rising healthcare costs and limited hospital budgets, these results suggest that EmboTrap proves to be an evidence-based economical choice of stent retriever for hospitals and healthcare systems.
Summary points
The economic impact of achieving improved functional outcomes was explored across three commonly used stent retrievers (SRs; EmboTrap, Trevo and Solitaire) using functional outcome data reported in MASTRO I, a recent living systematic review and meta-analysis.
Based on the proportion of patients who achieved a 90-day modified Rankin Scale score of 0–2 as reported in MASTRO I, costs associated with short-term index hospitalization, long-term one-year post-hospitalization costs and total costs (short-term index hospitalization and 1-year post-hospitalization costs) were estimated for each device.
Total (short- and long-term) cost savings were reported from the perspective of the US, Canada, the UK, Sweden, Germany, France, Italy, Spain, Belgium and The Netherlands healthcare systems.
Across all countries studied, patients treated with EmboTrap during mechanical thrombectomy (MT) were associated with the lowest costs, followed by patients treated with Trevo, and then those treated with Solitaire. The reduced costs were likely due to the shorter length of stay and the lower healthcare resource use 1-year post-stroke associated with achieving 90-day good functional outcomes.
Over the 1-year period, the greater savings in total cost were reported for EmboTrap versus Solitaire, followed by EmboTrap versus Trevo, with Trevo versus Solitaire having the smallest cost savings.
In summary, the use of EmboTrap resulted in the lowest per-patient costs, resulting in cost savings relative to Trevo and Solitaire, due to the proportion of patients achieving good functional outcomes.
The results of this analysis provides additional information that payers and clinicians may leverage in making evidence-based decisions to inform SR selection during MT.
The results of this study should be interpreted in the context of the limitations of the analysis including heterogeneity in the MASTRO I dataset, the inability to account for all potential variables that may influence MT costs, use of potentially outdated economic data, and that adjunctive device use was not able to be fully evaluated in the analysis.
Further research is needed that examines the relationship between functional outcomes and short- and long-term costs in stroke treatment.
Supplementary Material
Acknowledgements
The authors acknowledge S Ikeme, M Kocaman, A Sweeney, F Pinckaers and members of the Value & Evidence team at EVERSANA for contributing to the study conception, imputation and review of input parameters, model review, and manuscript writing and review.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2024-0216
Author contributions
T Andersson, H Nordmeyer, W Brinjikji and OO Zaida contributed to conception of the study, critically reviewed the model and manuscript, and provided final approval of the version for publication. E Kottenmeier and S Scheffler contributed to the conception and design of the study, collection, imputation and review of input parameters, model critique and validation, analysis and interpretation of results, manuscript writing and review and provided final approval of the version for publication. M Kabiri contributed to conception and design of the study, full model development, model review, validation and correction, analysis, interpretation of results, manuscript writing and review, and provided final approval of the version for publication. PA Brouwer and M Mirza contributed to the conception of the study, model review, parameter critique and validation, model critique and validation, interpretation of results, manuscript review and provided final approval of the version for publication.
Financial disclosure
This study was sponsored by Johnson & Johnson MedTech. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
T Andersson reports consulting fees for Anaconda, Johnson & Johnson MedTech, Neuravi, Rapid Medical, Optimize Neurovascular and holds equity in Ceroflo. H Nordmeyer reports consulting fees from Acandis, Balt, Johnson & Johnson MedTech, Rapid Medical. W Brinjikji reports consulting fees for Medtronic, Stryker, Imperative Care, Microvention, MIVI Neurovascular, Johnson & Johnson MedTech, Asahi, and Balt; holds equity in Nested Knowledge, Superior Medical Editors, Piraeus Medical, Sonoris Medical, and MIVI Neurovascular; receives royalties from Medtronic and Balloon Guide Catheter Technology; and serves in a leadership or fiduciary role for MIVI Neurovascular, Marblehead Medical LLC, Interventional Neuroradiology (Editor in Chief), Piraeus Medical, and WFITN. E Kottenmeier, M Kabiri, S Scheffler, PA Brouwer, M Mirza all report employment by Johnson & Johnson MedTech. OO Zaidat reports consulting fees for Stryker, Medtronic, Johnson & Johnson MedTech, and Penumbra; research grants from Stryker, Medtronic, Johnson & Johnson MedTech, Penumbra, and Genentech; in addition, OO Zaidat had a patent for ischemic stroke issued. The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editorial support were provided by EVERSANA and were funded by Johnson & Johnson MedTech.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Tsao CW, Aday AW, Almarzooq ZI et al. Heart Disease and Stroke Statistics-2023 Update: a report from the American Heart Association. Circulation 147(8), e93–e621 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. The top 10 causes of death. (Accessed: February 2024). https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 3.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20(10), 795–820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz Martínez MY, Alejandra Cantú Saldaña K, Antonio Ibarra Arias JJ. Available therapeutics after a stroke: current and promising options. New Insight into Cerebrovascular Diseases - An Updated Comprehensive Review (2020). [Google Scholar]
- 5.Gbiri CA, Olawale OA, Isaac SO. Stroke management: informal caregivers' burdens and strians of caring for stroke survivors. Ann. Phys. Rehabil. Med. 58(2), 98–103 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Dunbar SB, Khavjou OA, Bakas T et al. Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035: a policy statement from the American Heart Association. Circulation 137(19), e558–e577 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Martin SS, Aday AW, Almarzooq ZI et al. 2024 Heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation 149(8), e347–e913 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyas MV, Fang J, de Oliveira C et al. Attributable costs of stroke in Ontario, Canada and their variation by stroke type and social determinants of health. Stroke 54(11), 2824–2831 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Luengo-Fernandez R, Violato M, Candio P, Leal J. Economic burden of stroke across Europe: a population-based cost analysis. Eur. Stroke J. 5(1), 17–25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The country-specific per-patient cost for hospital day following stroke for the European countries were leveraged from Luengo-Fernandez et al.
- 10.Beumer D, Mulder MJHL, Saiedie G et al. Occurrence of intracranial large vessel occlusion in consecutive, non-referred patients with acute ischemic stroke. Neurovasc. Imaging 2(1), 1–6 (2016). [Google Scholar]
- 11.Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front. Neurol. 8, 651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith WS, Lev MH, English JD et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 40(12), 3834–3840 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers WJ, Rabinstein AA, Ackerson T et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12), e344–e418 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Turc G, Bhogal P, Fischer U et al. European Stroke Organisation (ESO) – European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J. NeuroIntervent. Surg. 15(8), e8 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Heran M, Lindsay P, Gubitz G et al. Canadian stroke best practice recommendations: Acute stroke management, 7(th) Edition Practice Guidelines Update, 2022. Can. J. Neurol. Sci. 51(1), 1–31 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Lambrinos A, Schaink AK, Dhalla I et al. Mechanical thrombectomy in acute ischemic stroke: a systematic review. Can. J. Neurol. Sci. 43(4), 455–460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candio P, Violato M, Leal J, Luengo-Fernandez R. Cost-effectiveness of mechanical thrombectomy for treatment of nonminor ischemic stroke across Europe. Stroke 52(2), 664–673 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shireman TI, Wang K, Saver JL et al. Cost-effectiveness of Solitaire stent retriever thrombectomy for acute ischemic stroke: Results from the SWIFT-PRIME Trial (Solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke). Stroke 48(2), 379–387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed SU, Chen X, Peeling L, Kelly ME. Stentrievers: an engineering review. Interv. Neuroradiol. 29(2), 125–133 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidat OO, Ikeme S, Sheth SA et al. MASTRO I: meta-Analysis and systematic review of thrombectomy stent retriever outcomes: comparing functional, safety and recanalization outcomes between EmboTrap, Solitaire and Trevo in acute ischemic stroke. J. Comp. Eff. Res. 12(5), e230001 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• MASTRO I was a meta-analysis and systematic review of 51 studies (9804 patients), comparing outcomes between EmboTrap, Solitaire and Trevo stent retrievers. The study found that use of EmboTrap during mechanical thrombectomy was associated with a significantly higher rate of favorable 90-day outcomes (modified Rankin Scale [mRS] 0–2) at 57.4%, compared with 45.3% for Solitaire (p < 0.001) and 50.0% for Trevo (p = 0.013). Pooled estimates for achieving functional independence (mRS 0–2), functional dependence (mRS 3–5), and mortality (mRS 6) at 90 days were obtained from MASTRO I.
- 21.Mirza M, McCarthy R, Gilvarry M. EP64 Systematic review and analysis of pre-clinical side-by-side comparisons of EmboTrap versus Solitaire performance. J. Neurointerv. Surg. 13(Suppl. 2), A26 (2021). [Google Scholar]
- 22.Zaidat OO, Bozorgchami H, Ribó M et al. Primary results of the multicenter ARISE II study (Analysis of revascularization in ischemic stroke with EmboTrap). Stroke 49(5), 1107–1115 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Kim SE, Lee H, Kim JY et al. Three-month modified Rankin Scale as a determinant of 5-year cumulative costs after ischemic stroke: an analysis of 11,136 patients in Korea. Neurology 94(9), e978–e991 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Dewilde S, Annemans L, Peeters A et al. Modified Rankin scale as a determinant of direct medical costs after stroke. Int. J. Stroke 12(4), 392–400 (2017). [DOI] [PubMed] [Google Scholar]; •• The average length of stay (LOS) for mRS 0–2 and 3–5 were calculated using the LOS aggregated by mRS score (0–5) reported in DeWilde et al.
- 25.Simpson KN, Simpson AN, Mauldin PD et al. Observed cost and variations in short term cost-effectiveness of therapy for ischemic stroke in interventional management of stroke (IMS) III. J. Am. Heart. Assoc. 6(5), e004513 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The country-specific per-patient cost for hospital day following stroke for the US was leveraged from Simpson et al.
- 26.Mittmann N, Seung SJ, Hill MD et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can. J. Neurol. Sci. 39(6), 793–800 (2012). [DOI] [PubMed] [Google Scholar]; • The country-specific per-patient cost for hospital day following stroke and the long-term cost inputs for Canada was leveraged from Mittmann et al.
- 27.Lobotesis K, Veltkamp R, Carpenter IH et al. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischemic stroke in the UK. J. Med. Econ. 19(8), 785–794 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Oliveira Gonçalves ASO, Rohmann JL, Piccininni M et al. Economic evaluation of a mobile stroke unit service in Germany. Ann. Neurol. 93(5), 942–951 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Barral M, Armoiry X, Boudour S et al. Cost-effectiveness of stent-retriever thrombectomy in large vessel occlusion strokes of the anterior circulation: analysis from the French societal perspective. Rev. Neurol. (Paris) 176(3), 180–188 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Fattore G, Torbica A, Susi A et al. The social and economic burden of stroke survivors in Italy: a prospective, incidence-based, multi-centre cost of illness study. BMC Neurol. 12, 137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Andrés-Nogales F, Álvarez M, de Miquel M et al. Cost-effectiveness of mechanical thrombectomy using stent retriever after intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in the treatment of acute ischaemic stroke due to large vessel occlusion in Spain. Eur. Stroke J. 2(3), 272–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekander I, Willers C, von Euler M et al. Relationship between functional disability and costs one and two years post stroke. PLoS One 12(4), e0174861 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinckaers FME, Grutters JPC, Huijberts I et al. Cost and utility estimates per modified rankin scale score up to 2 years post stroke: data to inform economic evaluations from a societal perspective. Value Health 27(4), 441–448 (2024). [DOI] [PubMed] [Google Scholar]
- 34.OECD. Purchasing power parities (PPP) (indicator). doi: (2017).
- 35.IMF Data. Country Indexes And Weight. (Accessed: October 2023). https://data.imf.org/regular.aspx?key=61015892 ; • When required, costs were inflated to 2023 values using the health-specific Consumer Price Indices from the International Monetary Fund.
- 36.Johnson BH, Bonafede MM, Watson C. Short- and longer-term health-care resource utilization and costs associated with acute ischemic stroke. Clinicoecon Outcomes Res. 8, 53–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel A, Berdunov V, Quayyum Z et al. Estimated societal costs of stroke in the UK based on a discrete event simulation. Age Ageing 49(2), 270–276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas-Noll J, Clua-Espuny JL, Lleixa-Fortuno M et al. The costs associated with stroke care continuum: a systematic review. Health Econ. Rev. 13(1), 32 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 319(10), 1024–1039 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Pożarowszczyk N, Kurkowska-Jastrzębska I, Sarzyńska-Długosz I, Nowak M, Karliński M. Reliability of the modified Rankin scale in clinical practice of stroke units and rehabilitation wards. Front Neurol. 14, 1064642 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials. Stroke 38(3), 1091–1096 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin. Interv. Aging 8, 201–211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broderick JP, Adeoye O, Elm J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke 48(7), 2007–2012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chye A, Hackett ML, Hankey GJ et al. Repeated measures of modified Rankin scale scores to assess functional recovery from stroke: AFFINITY study findings. Am. Heart J. 11(16), e025425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parameshwaran B, Cordato D, Parsons M et al. The benefit of endovascular thrombectomy for stroke on functional outcome is sustained at 12 months. Cerebrovasc. Dis. Extra 11(2), 81–86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunasekera L, Mitchell P, Dowling RJ, Bush S, Yan B. Functional recovery continues beyond 3 months post-basilar artery thrombectomy: a retrospective cohort study. CNS Neurosci. Ther. 29(8), 2171–2176 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Havenon A, Tirschwell DL, Heitsch L et al. Variability of the modified Rankin scale score between day 90 and 1 year after ischemic stroke. Neurol. Clin. Pract. 11(3), e239–e244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


