Abstract
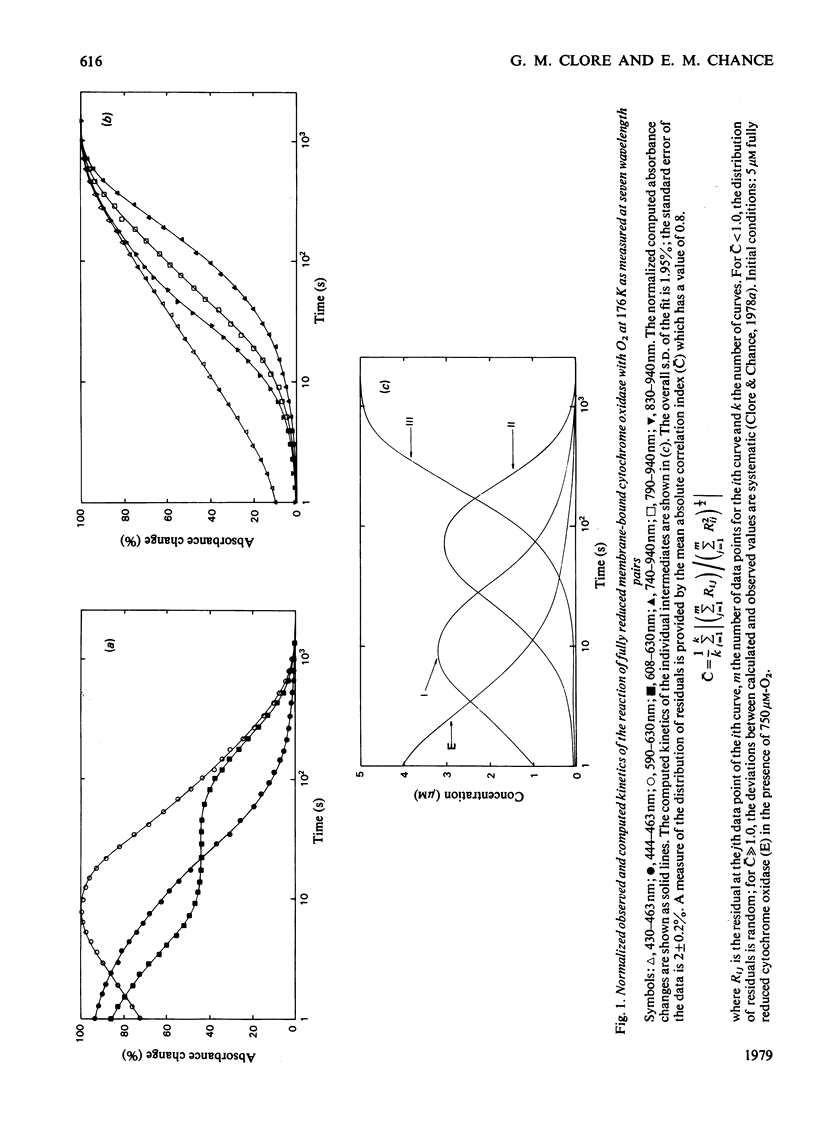
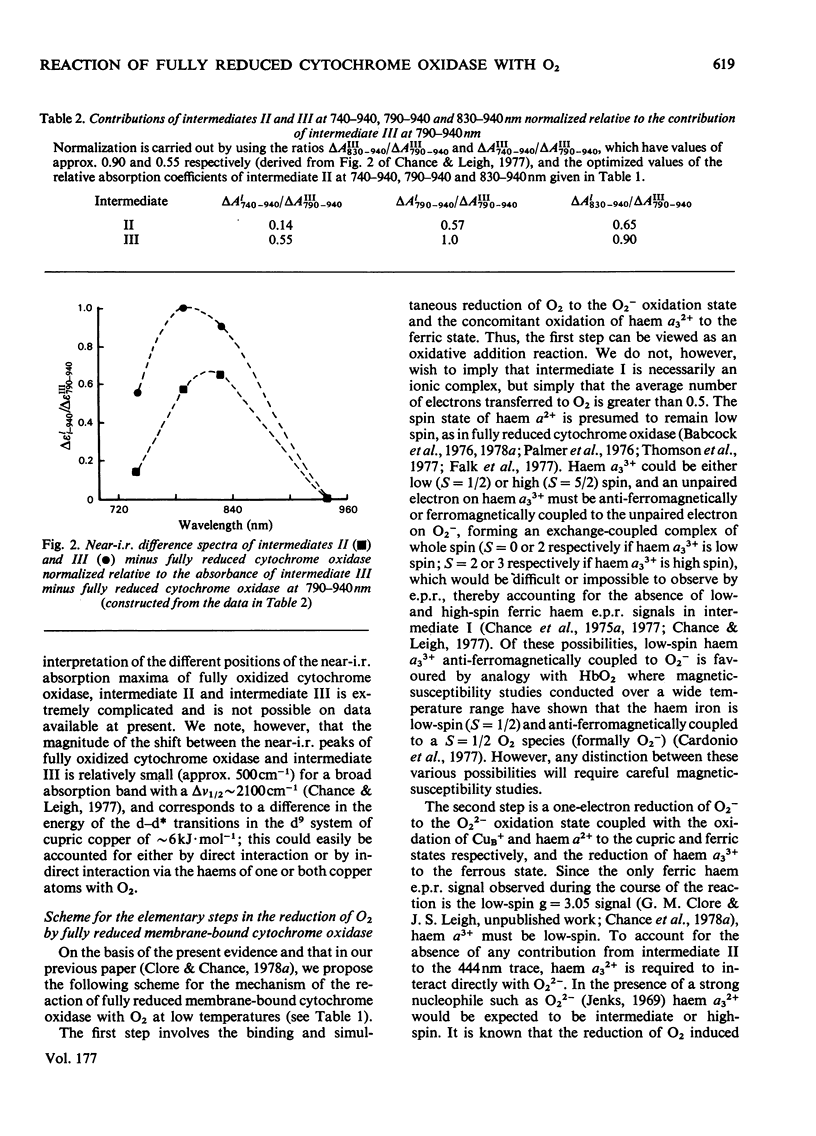
The kinetics of the reaction of fully reduced membrane-bound cytochrome oxidase with O2 obtained in the Soret, alpha and near-i.r. regions were analysed, and the contributions of the three intermediates of the reaction [Clore & Chance (1978) Biochem. J. 173, 799--810] to seven wavelength pairs (430--463, 444--463, 590--630, 608--630, 740--940, 790--940 and 830--940 nm) were determined. The nature of the intermediates is discussed on the basis of the data in the present paper together with data in the literature from optical wavelength scanning, e.p.r., i.r. and magnetic-susceptibility studies.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. The electronic state of heme in cytochrome oxidase II. Oxidation-reduction potential interactions and heme iron spin state behavior observed in reductive titrations. J Biol Chem. 1978 Apr 10;253(7):2400–2411. [PubMed] [Google Scholar]
- Caughey W. S., Barlow C. H., Maxwell J. C., Volpe J. A., Wallace W. J. Reactions of oxygen with hemoglobin, cytochrome c oxidase and other hemeproteins. Ann N Y Acad Sci. 1975 Apr 15;244:1–9. doi: 10.1111/j.1749-6632.1975.tb41517.x. [DOI] [PubMed] [Google Scholar]
- Cerdonio M., Congiu-Castellano A., Mogno F., Pispisa B., Romani G. L., Vitale S. Magnetic properties of oxyhemoglobin. Proc Natl Acad Sci U S A. 1977 Feb;74(2):398–400. doi: 10.1073/pnas.74.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Legallais V., Sorge J., Graham N. A versatile time-sharing multichannel spectrophotometer, reflectometer, and fluorometer. Anal Biochem. 1975 Jun;66(2):498–514. doi: 10.1016/0003-2697(75)90617-x. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr Oxygen intermediates and mixed valence states of cytochrome oxidase: infrared absorption difference spectra of compounds A, B, and C of cytochrome oxidase and oxygen. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4777–4780. doi: 10.1073/pnas.74.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr, Ingledew W. J., King T. E. Low-temperature kinetics of the reaction of oxygen and solubilized cytochrome oxidase. Biochem J. 1978 Jun 1;171(3):787–798. doi: 10.1042/bj1710787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Waring A., Leigh J. S., Jr Cytochrome c-cytochrome oxidase interaction at subzero temperatures. Biochim Biophys Acta. 1978 Jul 6;503(1):37–55. doi: 10.1016/0005-2728(78)90160-3. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of ferricyanide-pretreated mixed-valence-state membrane-bound cytochrome oxidase with oxygen at 173 K. Biochem J. 1978 Sep 1;173(3):811–820. doi: 10.1042/bj1730811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of fully reduced membrane-bound cytochrome oxidase with oxygen at 176K. Biochem J. 1978 Sep 1;173(3):799–810. doi: 10.1042/bj1730799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco C., Duprè S., Crifò C., Rotilio G., Cavallini D. Copper-catalyzed oxidation of thiomalic acid. Arch Biochem Biophys. 1971 Jun;144(2):496–502. doi: 10.1016/0003-9861(71)90354-7. [DOI] [PubMed] [Google Scholar]
- Denis M. The involvement of the fully oxidized state in cytochrome oxidase reaction with oxygen studied with the 655 and nm band as a probe. FEBS Lett. 1977 Dec 15;84(2):296–298. doi: 10.1016/0014-5793(77)80710-2. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Chance B. Studies on the electron transport chain at subzero temperatures: electron transport at site 3. Arch Biochem Biophys. 1972 Jul;151(1):304–315. doi: 10.1016/0003-9861(72)90501-2. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Vänngård T., Angström J. Heme spin-states of cytochrome c oxidase derived from room temperature magnetic susceptibility measurements. FEBS Lett. 1977 Mar 15;75(1):23–27. doi: 10.1016/0014-5793(77)80044-6. [DOI] [PubMed] [Google Scholar]
- Gilmour M. V., Lemberg M. R., Chance B. Cytochrome oxidase and its derivatives. IX. Spectrophotometric studies on the rapid reaction of ferrous cytochrome c oxidase with molecular oxygen under conditions of complete and partial oxygenation. Biochim Biophys Acta. 1969 Jan 14;172(1):37–51. doi: 10.1016/0005-2728(69)90090-5. [DOI] [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- ORII Y., OKINUKI K. Studies on cytochrome a. IX. Significance of oxygenated cytochrome a in the cytochrome oxidase mechanism. J Biochem. 1963 Jun;53:489–499. doi: 10.1093/oxfordjournals.jbchem.a127728. [DOI] [PubMed] [Google Scholar]
- Palmer G., Babcock G. T., Vickery L. E. A model for cytochrome oxidase. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2206–2210. doi: 10.1073/pnas.73.7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. P. Variable-temperature magnetic-circular-dichroism spectra of cytochrome c oxidase and its derivatives. Biochem J. 1977 Aug 1;165(2):327–336. doi: 10.1042/bj1650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN GELDERB, SLATER E. C. TITRATION OF CYTOCHROME C OXIDASE WITH NADH AND PHENAZINE METHOSULPHATE. Biochim Biophys Acta. 1963 Aug 6;73:663–665. doi: 10.1016/0006-3002(63)90342-1. [DOI] [PubMed] [Google Scholar]
- Wever R., Van Drooge J. H., Muijsers A. O., Bakker E. P., Van Gelker B. F. The binding of carbon monoxide to cytochrome c oxidase. Eur J Biochem. 1977 Feb 15;73(1):149–154. doi: 10.1111/j.1432-1033.1977.tb11301.x. [DOI] [PubMed] [Google Scholar]
- Yammoto T., Palmer G. The valence and spin state of iron in oxyhemoglobin as inferred from resonance Raman spectroscopy. J Biol Chem. 1973 Jul 25;248(14):5211–5213. [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


