Abstract
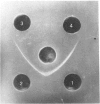
A thiamin-binding protein was isolated and characterized from chicken egg white by affinity chromatography on thiamin pyrophosphate coupled to aminoethyl-Sepharose. The high specificity of interaction between the thiamin-binding protein and the riboflavin-binding protein of the egg white, with a protein/protein molar ratio of 1.0, led to the development of an alternative procedure that used the riboflavin-binding protein immobilized on CNBr-activated Sepharose as the affinity matrix. The thiamin-binding protein thus isolated was homogeneous by the criteria of polyacrylamide-gel disc electrophoresis, double immunodiffusion and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, had a mol.wt. of 38,000 +/- 2000 and was not a glycoprotein. The protein bound [14C]thiamin was a molar ratio of 1.0, with dissociation constant (Kd) 0.3 micrometer.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Muto Y., Hosoya N. Vitamin A transport in chicken plasma: isolation and characterization of retinol-binding protein (RBP), prealbumin (PA), and RBP--PA complex. J Lipid Res. 1975 May;16(3):200–210. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Cutting J. A., Roth T. F. Changes in specific sequestration of protein during transport into the developing oocyte of the chicken. Biochim Biophys Acta. 1973 Apr 16;298(4):951–955. doi: 10.1016/0005-2736(73)90398-2. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Emtage J. S. Vitamin D in the avian egg. Its molecular identity and mechanism of incorporation into yolk. Biochem J. 1976 Dec 15;160(3):671–682. doi: 10.1042/bj1600671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 1. THE USE OF (14-C)BIOTIN FOR KINETIC STUDIES AND FOR ASSAY. Biochem J. 1963 Dec;89:585–591. doi: 10.1042/bj0890585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. Purification and evidence for the identity of chicken plasma and egg yolk retinol-retinol binding protein-prealbumin complex. Dev Biol. 1976 Jul 1;51(1):1–9. doi: 10.1016/0012-1606(76)90117-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Matsuura A., Iwashima A., Nose Y. Purification of thiamine-binding protein from Escherichia coli. Biochem Biophys Res Commun. 1973 Mar 5;51(1):241–246. doi: 10.1016/0006-291x(73)90534-2. [DOI] [PubMed] [Google Scholar]
- Meslar H. W., Camper S. A., White H. B., 3rd Biotin-binding protein from egg yolk. A protein distinct from egg white avidin. J Biol Chem. 1978 Oct 10;253(19):6979–6982. [PubMed] [Google Scholar]
- Murthy U. S., Adiga P. R. Oestrogen induction of riboflavin-binding protein in immature chicks. Nature of the secretory protein. Biochem J. 1978 Feb 15;170(2):331–335. doi: 10.1042/bj1700331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy U. S., Adiga P. R. Riboflavin-binding protein of hen's egg : purification & radioimmunoassay. Indian J Biochem Biophys. 1977 Jun;14(2):118–124. [PubMed] [Google Scholar]
- Murthy U. S., Podder S. K., Adiga P. R. The interaction of riboflavin with a protein isolated from hen's egg white: a spectrofluorimetric study. Biochim Biophys Acta. 1976 May 20;434(1):69–81. doi: 10.1016/0005-2795(76)90036-2. [DOI] [PubMed] [Google Scholar]
- NABER E. C., CRAVENS W. W., BAUMANN C. A., BIRD H. R. The effect of thiamine analogs on embryonic development and growth of the chick. J Nutr. 1954 Dec 10;54(4):579–591. doi: 10.1093/jn/54.4.579. [DOI] [PubMed] [Google Scholar]
- Nishikimi M., Kyogoku Y. Flavin-protein interaction in egg white flavoprotein. J Biochem. 1973 Jun;73(6):1233–1242. doi: 10.1093/oxfordjournals.jbchem.a130196. [DOI] [PubMed] [Google Scholar]
- OSTROWSKI W., SKARZYNSKI B., ZAK Z. Isolation and properties of flavoprotein from the egg yolk. Biochim Biophys Acta. 1962 May 21;59:515–517. doi: 10.1016/0006-3002(62)90217-2. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- RHODES M. B., BENNETT N., FEENEY R. E. The flavoprotein-apoprotein system of egg white. J Biol Chem. 1959 Aug;234(8):2054–2060. [PubMed] [Google Scholar]
- Sonneborn D. W., Hansen H. J. Vitamin B12 binders of chicken serum and chicken proventriculus are immunologically similar. Science. 1970 May 1;168(3931):591–592. doi: 10.1126/science.168.3931.591. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J. A comparison of conalbumin and transferrin in the domestic fowl. Biochem J. 1962 May;83:355–364. doi: 10.1042/bj0830355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White H. B., 3rd, Dennison B. A., Della Fera M. A., Whitney C. J., McGuire J. C., Meslar H. W., Sammelwitz P. H. Biotin-binding protein from chicken egg yolk. Assay and relationship to egg-white avidin. Biochem J. 1976 Aug 1;157(2):395–400. doi: 10.1042/bj1570395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter W. P., Buss E. G., Clagett C. O., Boucher R. V. The nature of the biochemical lesion in avian renal riboflavinuria. I. Effect of genotype on renal riboflavin metabolism. Comp Biochem Physiol. 1967 Sep;22(3):889–896. doi: 10.1016/0010-406x(67)90779-7. [DOI] [PubMed] [Google Scholar]
- Yusko S. C., Roth T. F. Binding to specific receptors on oocyte plasma membranes by serum phosvitin-lipovitellin. J Supramol Struct. 1976;4(1):89–97. doi: 10.1002/jss.400040109. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]