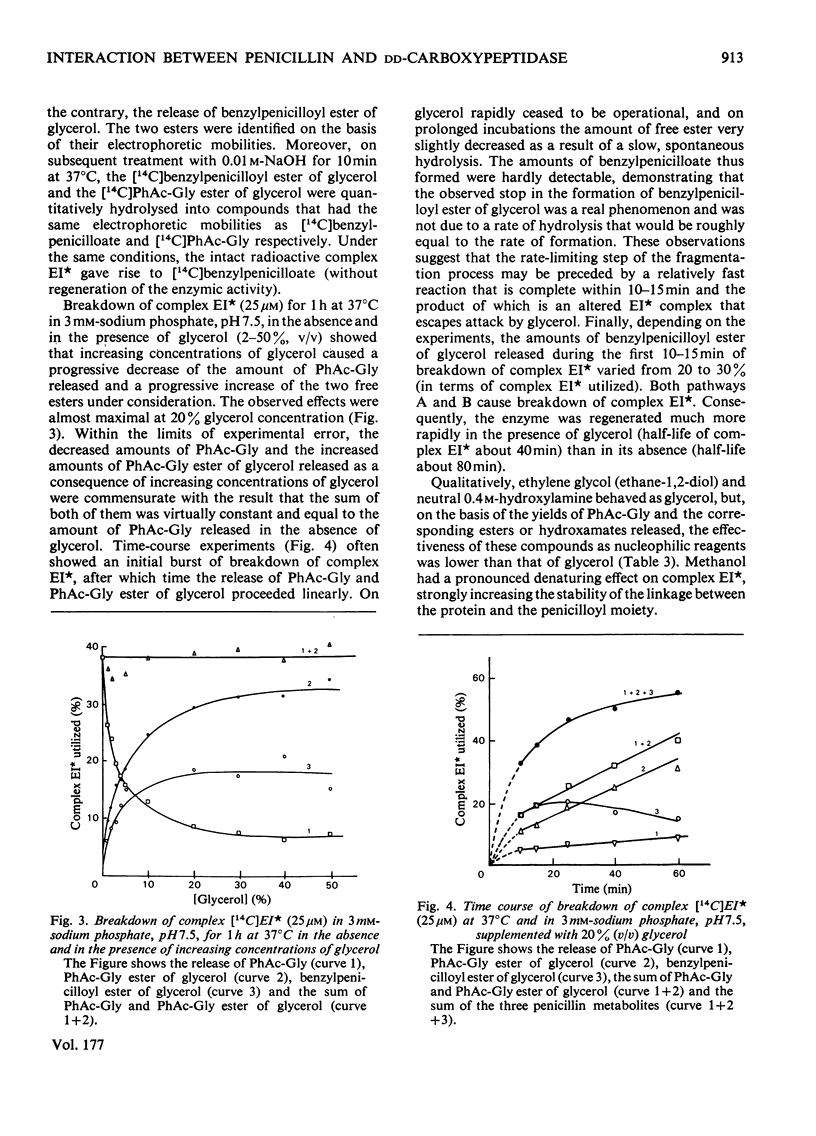
Abstract
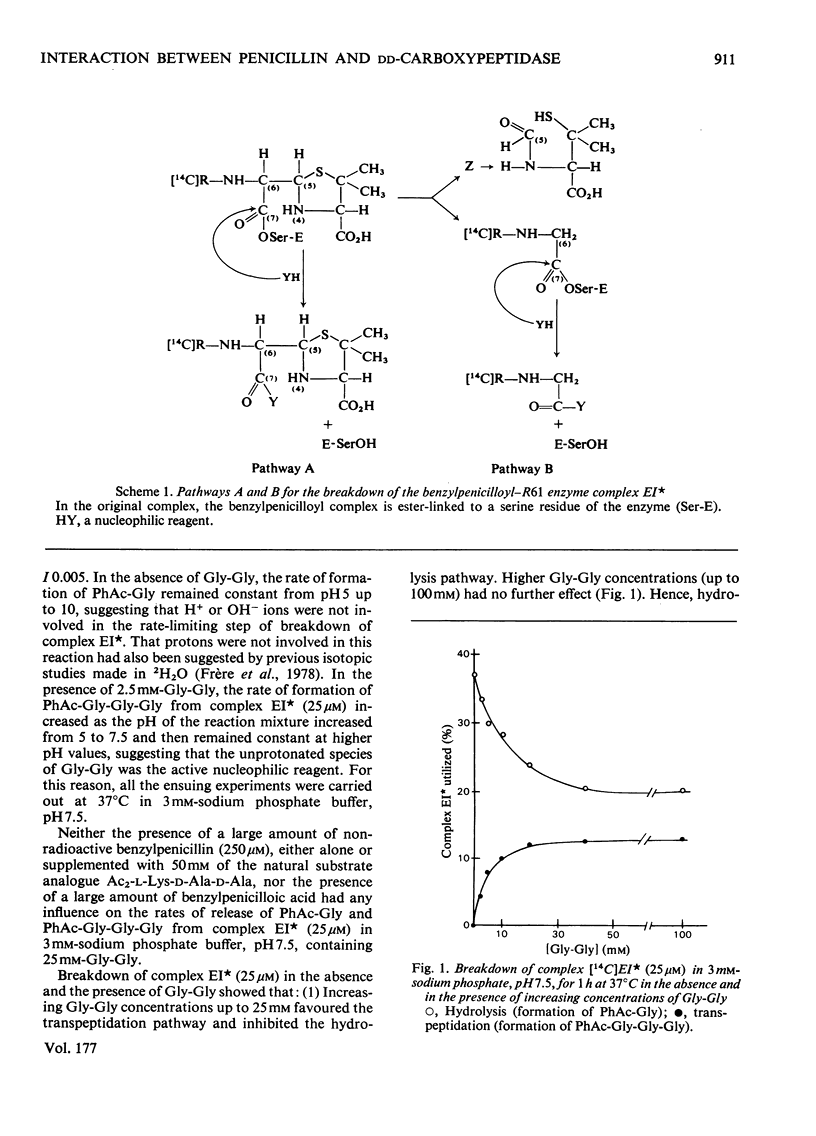
Serine is one of the enzyme residues with which benzylpenicillin collides as a result of its binding to the Streptomyces strain-R61 DD-carboxypeptidase-transpeptidase enzyme. Nucleophilic attack occurs on C(7) of the bound antibiotic molecule with formation of a benzylpenicilloyl-serine ester linkage, i.e. formation of the benzylpenicilloyl-enzyme EI complex. To reject the bound penicilloyl moiety and consequently to recover its initial activities, the strain-R61 enzyme has developed two possible mechanisms. Pathway A is a direct attack of the serine ester linkage by an exogenous nucleophile, resulting in the transfer of the benzylpenicilloyl moiety to this nucleophile. In pathway B, the benzylpenicilloyl moiety is first fragmented by C(5)-C(6) cleavage and the enzyme-bound phenylacetylglycyl residue thus produced is in turn transferred to the nucleophile. Pathway B occurs with water, glycylglycine and other amino compounds. Both pathways A and B occur with glycerol, other ROH nucleophiles and neutral hydroxylamine. The nucleophilic attacks are enzyme-catalysed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaens P., Meesschaert B., Frère J. M., Vanderhaeghe H., Degelaen J., Ghuysen J. M., Eyssen H. Stability of D-5,5-dimethyl-delta2-thiazoline-4-carboxylic acid in relation to its possible occurrence as a degradation product of penicillin by the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61 and the membrane-bound dd-carboxypeptidase from Bacillus stearothermophilus. J Biol Chem. 1978 May 25;253(10):3660–3665. [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Dusart J., Leyh-Bouille M., Ghuysen J. M. The peptidoglycan crosslinking enzyme system in Streptomyces strains R61, K15 and rimosus. Kinetic coefficients involved in the interactions of the membrane-bound transpeptidase with peptide substrates and beta-lactam antibiotics. Eur J Biochem. 1977 Nov 15;81(1):33–44. doi: 10.1111/j.1432-1033.1977.tb11924.x. [DOI] [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Degelaen J., Loffet A., Perkins H. R. Fragmentation of benzylpenicillin after interaction with the exocellular DD-carboxypeptidase-transpeptidases of Streptomyces R61 and R39. Nature. 1975 Nov 13;258(5531):168–170. doi: 10.1038/258168a0. [DOI] [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Vanderhaeghe H., Adriaens P., Degelaen J., De Graeve J. Fate of thiazolidine ring during fragmentation of penicillin by exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Nature. 1976 Apr 1;260(5550):451–454. doi: 10.1038/260451a0. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Duez C., Ghuysen J. M., Vandekerkhove J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1976 Nov;70(1):257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Iwatsubo M. Kinetics of interaction between the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61 and beta-lactam antibiotics. A choice of models. Eur J Biochem. 1975 Sep 15;57(2):343–351. doi: 10.1111/j.1432-1033.1975.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Kinetics of concomitant transfer and hydrolysis reactions catalysed by the exocellular DD-carboxypeptidase-transpeptidase of streptomyces R61. Biochem J. 1973 Nov;135(3):483–492. doi: 10.1042/bj1350483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Molecular weight and amino acid composition of the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Biochem J. 1973 Nov;135(3):463–468. doi: 10.1042/bj1350463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., de Graeve J. Fragmentation of penicillin catalysed by the exocellular DD-carboxypeptidase-transpeptidase of Streptomyces strain r61. Isotopic study of hydrogen fixation on carbon 6. FEBS Lett. 1978 Apr 1;88(1):147–150. doi: 10.1016/0014-5793(78)80628-0. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Nishino T., Willoughby E., Strominger J. L. Hydroxylaminolysis of penicillin binding componenets is enzymatically catalyzed. J Biol Chem. 1977 Nov 10;252(21):7525–7529. [PubMed] [Google Scholar]
- Marquet A., Dusart J., Ghuysen J. M., Perkins H. R. Membrane-bound transpeptidase and penicillin binding sites in Streptomyces strain R61. Eur J Biochem. 1974 Aug 1;46(3):515–523. doi: 10.1111/j.1432-1033.1974.tb03645.x. [DOI] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M., Frére J. M., Leyh-Bouille M., Ghuysen J. M. Streptomyces DD-carboxypeptidases as transpeptidases. The specificity for amino compounds acting as carboxyl acceptors. Biochem J. 1973 Apr;131(4):707–718. doi: 10.1042/bj1310707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilf W., Frère P., Frère J. M., Martin H. H., Ghuysen J. M., Adriaens P., Meesschaert B. Interaction between penicillin and the DD-carboxypeptidase of the unstable L-form of Proteus mirabilis strain 19. Eur J Biochem. 1978 Apr 17;85(2):325–330. doi: 10.1111/j.1432-1033.1978.tb12242.x. [DOI] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Zeiger A. R., Frère J. M., Ghuysen J. M., Perkins H. R. A donor-acceptor substrate of the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1975 Apr 1;52(2):221–225. doi: 10.1016/0014-5793(75)80810-6. [DOI] [PubMed] [Google Scholar]


