Abstract
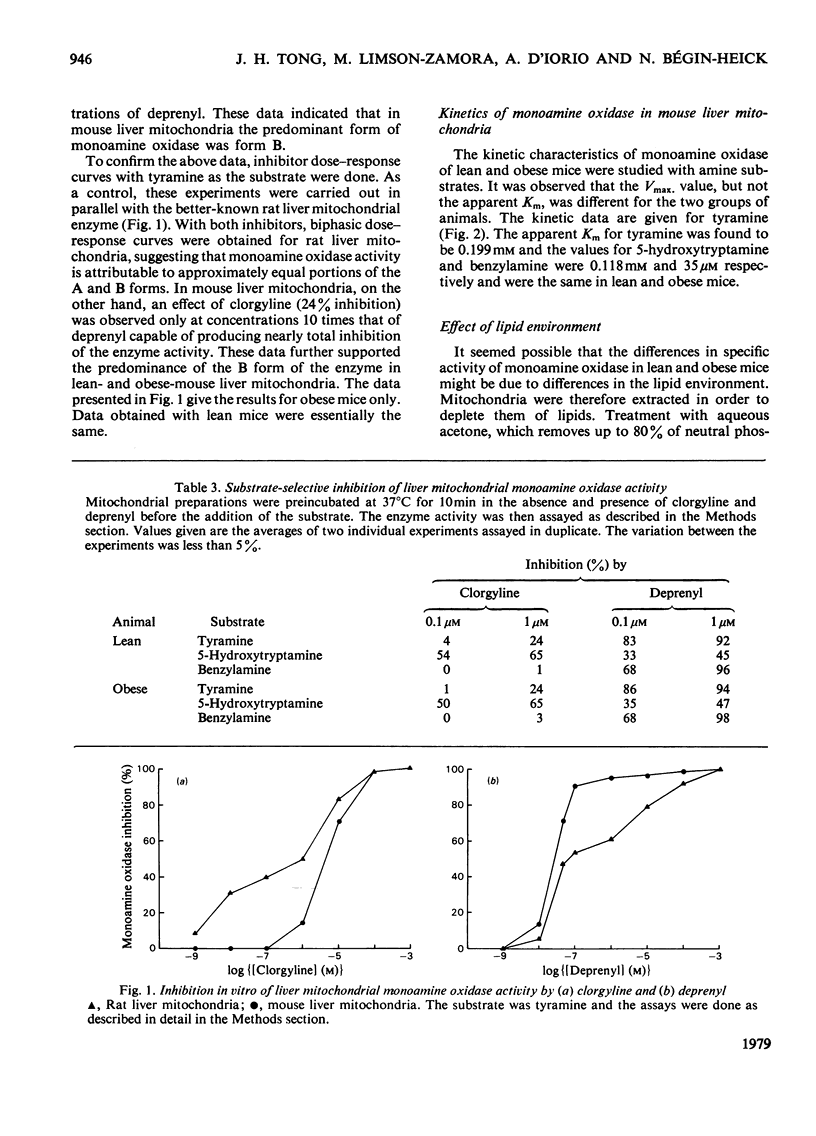
1. The specific activity of monoamine oxidase was found to be greater in liver mitochondria from ob/ob mice than from lean mice. The activities of marker enzymes were similar in both tissues. 2. Experiments with various substrates (5-hydroxytryptamine, benzylamine and tyramine) and inhibitors (clorgyline and deprenyl) indicated that, unlike rat liver mitochondria, mouse liver mitochondria contain a predominance of the B-form of monoamine oxidase. 3. The Km values for lean and ob/ob mice were the same for any given substrate and were in the increasing order 5-hydroxytryptamine less than tyramine less than benzylamine. Vmax. was approximately 50% greater in obese than in lean mice. 4. Extraction of liver mitochondria with acetone/water or acetone/water/NH3 to remove lipids decreased the enzyme activity relatively more in obese- than in lean-mice preparations, but residual activity was the same in both preparations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Huang D., Cuatrecasas P. The defect in insulin receptors in obese-hyperglycemic mice: a probable accompaniment of more generalized alterations in membrane glycoproteins. Biochem Biophys Res Commun. 1975 May 19;64(2):566–573. doi: 10.1016/0006-291x(75)90359-9. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Egashira T., Ekstedt B., Oreland L. Inhibition by clorgyline and deprenyl of the different forms of monoamine oxidase in rat liver mitochondria. Biochem Pharmacol. 1976 Dec 1;25(23):2583–2586. doi: 10.1016/0006-2952(76)90512-8. [DOI] [PubMed] [Google Scholar]
- Ekstedt B., Oreland L. Effect of lipid-depletion on the different forms of monoamine oxidase in rat liver mitochondria. Biochem Pharmacol. 1976 Jan 15;25(2):119–124. doi: 10.1016/0006-2952(76)90277-x. [DOI] [PubMed] [Google Scholar]
- Ekstedt B. Substrate specificity of the different forms of monoamine oxidase in rat liver mitochondria. Biochem Pharmacol. 1976 May 15;25(10):1133–1138. doi: 10.1016/0006-2952(76)90359-2. [DOI] [PubMed] [Google Scholar]
- Feldman J. M., Henderson J. H. Monoamine oxidase, catechol-O-methyltransferase, and norepinephrine levels in mice with the hereditary obese-hyperglycemic syndrome. Diabetes. 1978 Apr;27(4):389–395. doi: 10.2337/diab.27.4.389. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The nature of the electrophoretically separable multiple forms of rat liver monoamine oxidase. Biochem J. 1973 Sep;135(1):173–186. doi: 10.1042/bj1350173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laverty R., Browne B. J., Callingham B. A. Letter: Substrate specificity of monoamine oxidase activity in various mouse and rat tissues. J Pharm Pharmacol. 1973 Dec;25(12):1001–1002. doi: 10.1111/j.2042-7158.1973.tb09994.x. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Lovenberg W., Keiser H., Sjoerdsma A. Effects of drugs on human blood platelet and plasma amine oxidase activity in vitro and in vivo. Biochem Pharmacol. 1968 Jan;17(1):109–119. doi: 10.1016/0006-2952(68)90163-9. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. L., Glassman A. T. Multiple binding sites of human brain and liver monoamine oxidase: substrate specificities, selective inhibitions, and attempts to separate enzyme forms. J Neurochem. 1977 Dec;29(6):987–997. doi: 10.1111/j.1471-4159.1977.tb06502.x. [DOI] [PubMed] [Google Scholar]


