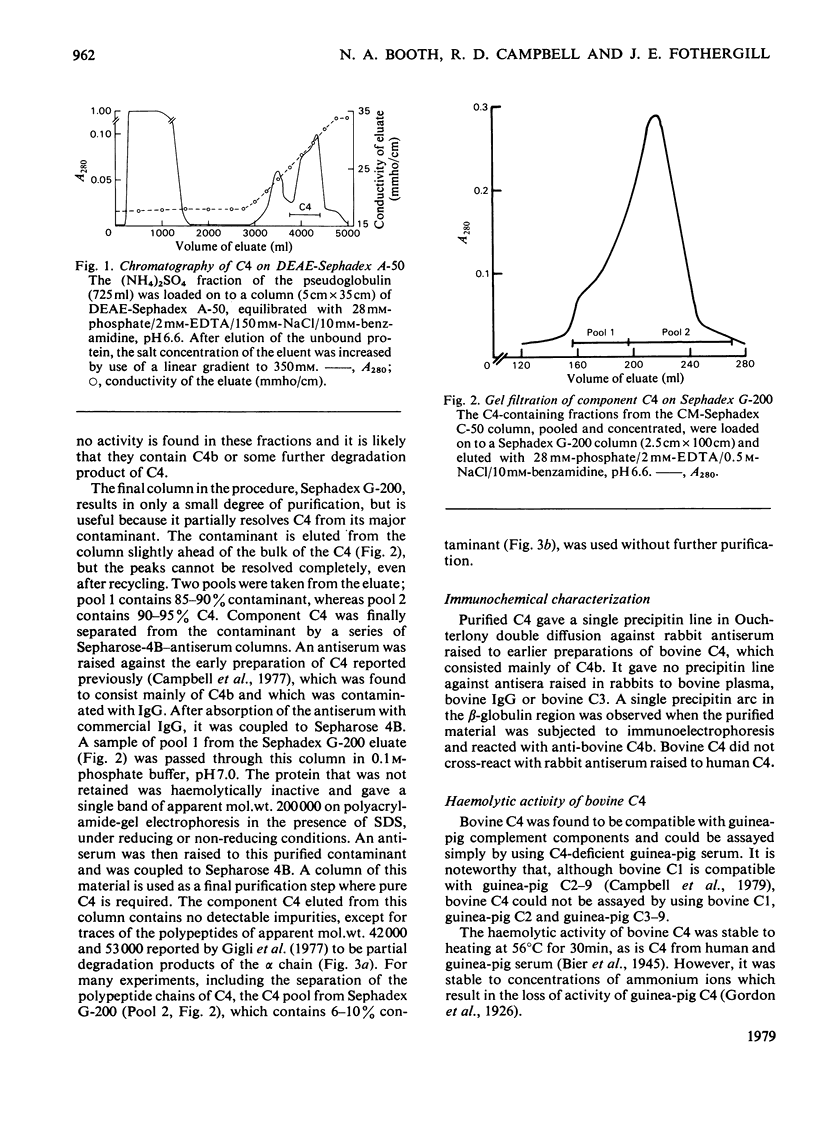
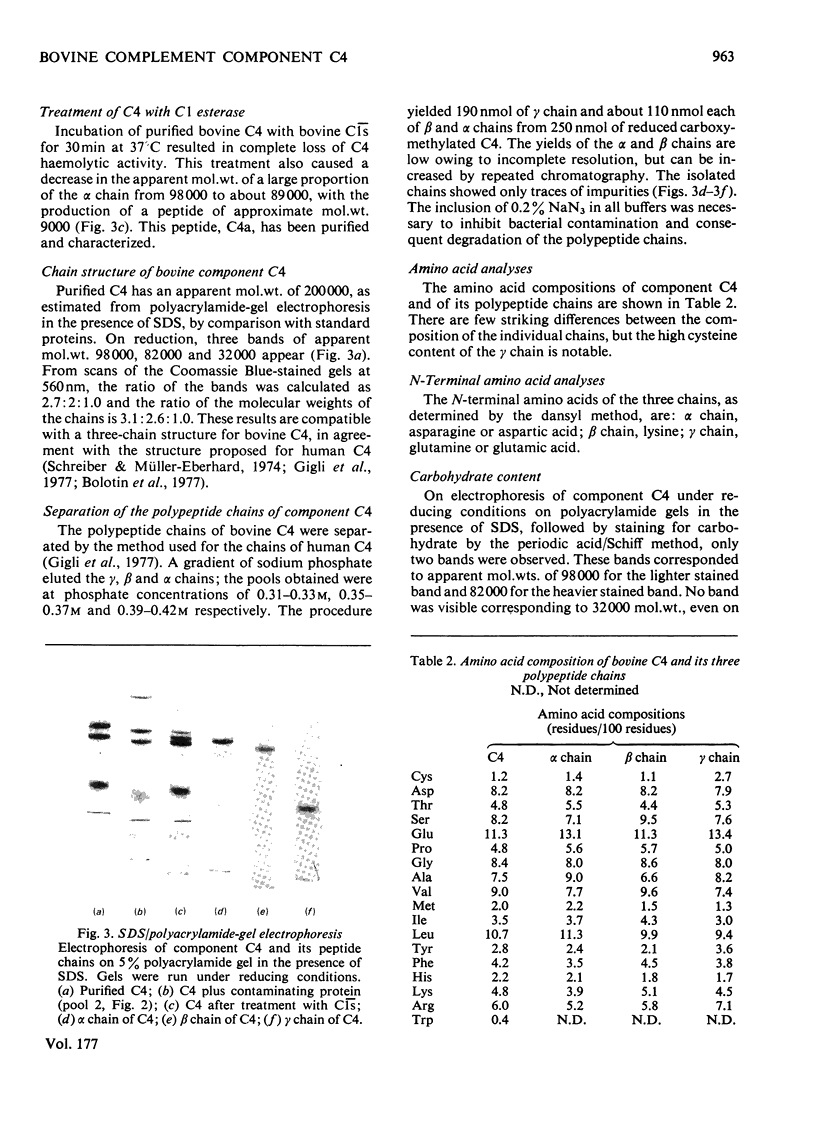
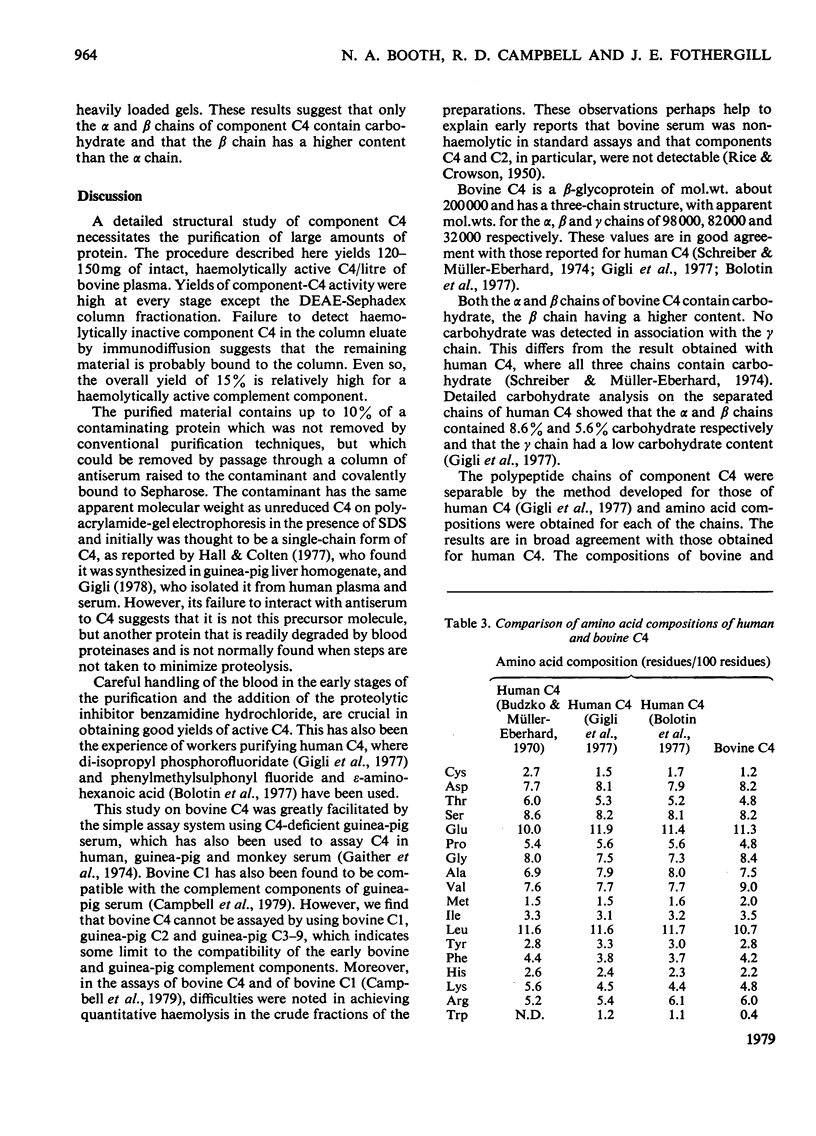
Abstract
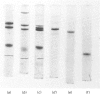
The fourth component of complement, C4, was isolated from bovine plasma in high yield, by using simple purification techniques. The protein, like human component C4, is a beta-globulin with a mol.wt. of about 200 000 and consists of three polypeptide chains, alpha, beta and gamma, with apparent mol. wts. of 98 000, 82 000 and 32 000 respectively. The chains of C4 have been separated by methods previously used for human C4. Their amino acid compositions are very similar to those of the human component, but differences in carbohydrate distribution have been observed. The haemolytic activity of bovine C4 is totally destroyed by incubation with bovine C1s, the activated subcomponent of the first component of complement. Component C4, treated in this way, was shown to be cleaved in the alpha chain, which was decreased in mol.wt. by about 9000, corresponding to the removal of subcomponent C4a.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokisch V. A., Dierich M. P., Mūller-Eberhard H. J. Third component of complement (C3): structural properties in relation to functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1989–1993. doi: 10.1073/pnas.72.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Bray D., Brownlee S. M. Peptide mapping of proteins from acrylamide gels. Anal Biochem. 1973 Sep;55(1):213–221. doi: 10.1016/0003-2697(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Müller-Eberhard H. J. Cleavage of the fourth component of human complement (C4) by C1 esterase: isolation and characteristics of the low molecular weight product. Immunochemistry. 1970 Feb;7(2):227–234. doi: 10.1016/0019-2791(70)90158-8. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Booth N. A., Fothergill J. E. Purification and characterization of subcomponent C1q of the first component of bovine complement. Biochem J. 1979 Feb 1;177(2):531–540. doi: 10.1042/bj1770531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Fernandez H. N., Huglij T. E. Chemical evidence for common genetic ancestry of complement components C3 and C5. J Biol Chem. 1977 Mar 10;252(5):1826–1828. [PubMed] [Google Scholar]
- Fothergill J. E., Anderson W. H. A molecular approach to the complement system. Curr Top Cell Regul. 1978;13:259–311. doi: 10.1016/b978-0-12-152813-3.50012-4. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Alling D. W., Frank M. M. A new one-step method for the functional assay of the fourth component (C4) of human and guinea pig complement. J Immunol. 1974 Aug;113(2):574–583. [PubMed] [Google Scholar]
- Gigli I. A single chain precursor of C4 in human serum. Nature. 1978 Apr 27;272(5656):836–837. doi: 10.1038/272836a0. [DOI] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Whitehead H. R., Wormall A. The Action of Ammonia on Complement. The Fourth Component. Biochem J. 1926;20(5):1028–1035. doi: 10.1042/bj0201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. E., Colten H. R. Cell-free synthesis of the fourth component of guinea pig complement (C4): identification of a precursor of serum C4 (pro-C4). Proc Natl Acad Sci U S A. 1977 Apr;74(4):1707–1710. doi: 10.1073/pnas.74.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- MARES-GUIA M., SHAW E. STUDIES ON THE ACTIVE CENTER OF TRYPSIN. THE BINDING OF AMIDINES AND GUANIDINES AS MODELS OF THE SUBSTRATE SIDE CHAIN. J Biol Chem. 1965 Apr;240:1579–1585. [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., LEPOW I. H. C'1 ESTERASE EFFECT ON ACTIVITY AND PHYSICOCHEMICAL PROPERTIES OF THE FOURTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 May 1;121:819–833. doi: 10.1084/jem.121.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Molenaar J. L., Helder A. W., Müller M. A., Goris-Mulder M., Jonker L. S., Brouwer M., Pondman K. W. Physico-chemical and antigenic properties of human C3. Immunochemistry. 1975 May;12(5):359–364. doi: 10.1016/0019-2791(75)90001-4. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Tomar R. H., Taylor F. B., Jr Additional studies on human C5: development of a modified purification method and characterization of the purified product by polyacrylamide gel electrophoresis. Immunochemistry. 1972 Jul;9(7):709–723. doi: 10.1016/0019-2791(72)90015-8. [DOI] [PubMed] [Google Scholar]
- Patrick R. A., Taubman S. B., Lepow I. H. Cleavage of the fourth component of human complement (C4) by activated Cls. Immunochemistry. 1970 Feb;7(2):217–225. doi: 10.1016/0019-2791(70)90157-6. [DOI] [PubMed] [Google Scholar]
- Porter R. R. The eleventh Hopkins Memorial Lecture: The biochemistry of complement. Biochem Soc Trans. 1977;5(6):1659–1674. doi: 10.1042/bst0051659. [DOI] [PubMed] [Google Scholar]
- RICE C. E., CROWSON C. N. The interchangeability of the complement components of different animal species in the hemolysis of sheep erythrocytes sensitized with rabbit amboceptor. J Immunol. 1950 Aug;65(2):201–210. [PubMed] [Google Scholar]
- Schreiber R. D., Müller-Eberhard H. J. Fourth component of human complement: description of a three polypeptide chain structure. J Exp Med. 1974 Nov 1;140(5):1324–1335. doi: 10.1084/jem.140.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S., Stroud R. M. Cleavage products of C4b produced by enzymes in human serum. Immunochemistry. 1975 Dec;12(12):935–939. doi: 10.1016/0019-2791(75)90256-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]