Abstract
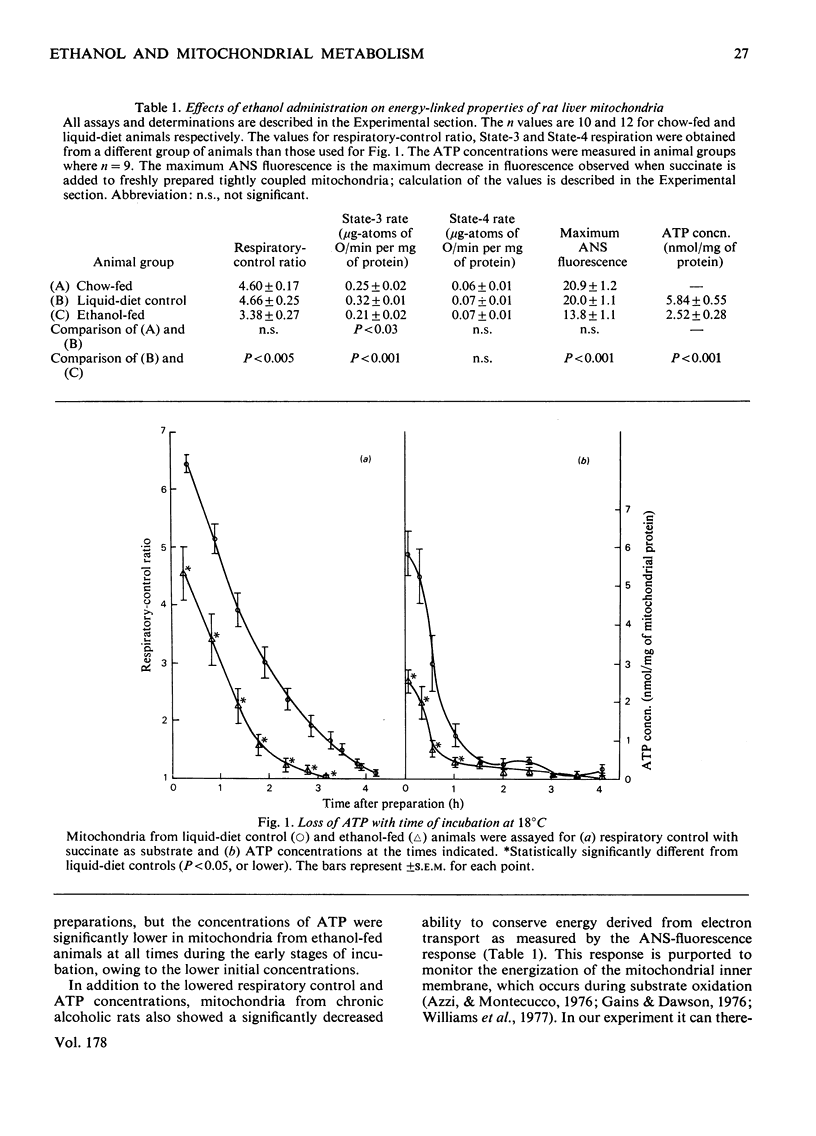
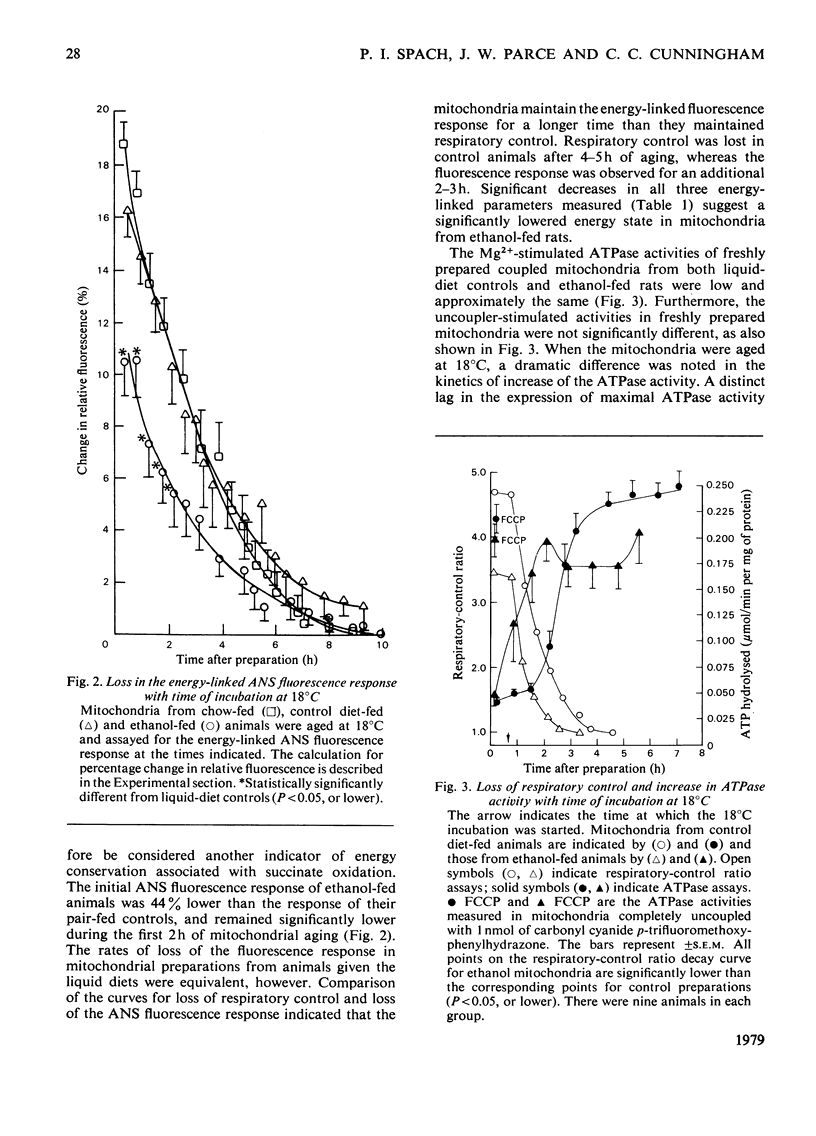
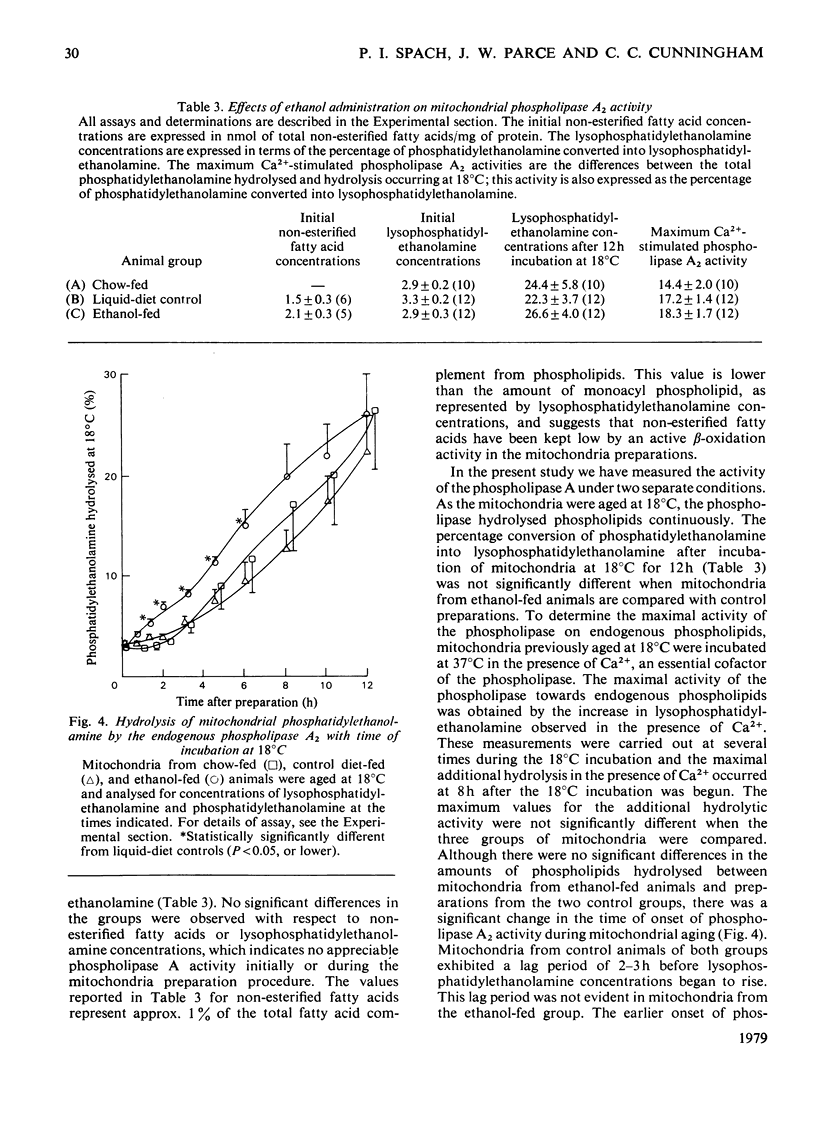
1. For a period of 31 days male rats were given a liquid diet containing 36% of its energy as ethanol. Liver mitochondria from these animals demonstrated lowered respiratory control with succinate as substrate, a diminished energy-linked anilinonaphthalene-sulphonic acid fluorescence response, and lowered endogenous ATP concentrations. The phospholipid/protein ratio in mitochondria from these animals was unchanged; only minor alterations in the phospholipid fatty acid composition were observed. 2. In experiments where mitochondria were incubated at 18 degrees C in iso-osmotic sucrose (aging experiments), the above energy-linked properties were lost at an earlier time in organelles from ethanol-fed animals. Phospholipase A2 acitivty was depressed in mitochondria from control animals until respiratory control was lost and ATP was depleted. In contrast, no lag in the expression of phospholipase activity was observed in mitochondria from ethanol-fed rats. This loss of control of the phospholipase resulted in an earlier degradation of membrane phospholipids under the conditions of the aging experiments. 3. The ATPase (adenosine triphosphatase) activities, measured in freshly prepared tightly coupled mitochondria and in organelles uncoupled with carbonyl cyanide p-trifluoromethoxyphenylhydrazone, were not significantly different in ethanol-fed and liquid-diet control animals. When the mitochondria were aged at 18 degrees C, the activity increased with time of incubation in organelles from both groups of animals. A lag was observed, however, as the ATPase activity increased in control preparations. This lag was not present as APTase activity increased in mitochondria from ethanol-fed animals. 4. The significantly lowered values observed for energy-linked functions with succinate as an energy source demonstrate that ethanol elicits an alteration in liver mitochondria that affects the site II-site III regions of the oxidative-phosphorylation system. The apparent lack of control of the phospholipase A2 and ATPase activities in mitochondria from ethanol-fed animals suggests that the membrane microenvironment of these enzymes has been altered such that they can exert their catabolic effects more readily under conditions of mild perturbation. The fatty acid analyses demonstrate that the observed alterations both in the energy-linked functions and in control of the phospholipase and ATPase are not mediated through changes in the acyl chain composition of bulk-phase phospholipids.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A. Redistribution of the electrical charge of the mitochondrial membrane during energy conservation. Biochem Biophys Res Commun. 1969 Oct 8;37(2):254–260. doi: 10.1016/0006-291x(69)90727-x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banks W. L., Jr, Kline E. S., Higgins E. S. Hepatic composition and metabolism after ethanol consumption in rats fed liquid purified diets. J Nutr. 1970 May;100(5):581–593. doi: 10.1093/jn/100.5.581. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Lieber C. S., Rubin E. Effects of chronic ethanol treatment of mitochondrial functions damage to coupling site I. Arch Biochem Biophys. 1974 Dec;165(2):560–569. doi: 10.1016/0003-9861(74)90283-5. [DOI] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Clandinin M. T. Fatty acid composition changes in mitochondrial membrances induced by dietary long chain fatty acids. FEBS Lett. 1976 Sep 15;68(1):41–44. doi: 10.1016/0014-5793(76)80400-0. [DOI] [PubMed] [Google Scholar]
- Datta A., Penefsky H. S. Interaction of fluorescent probes with submitochondrial particles during oxidative phosphorylation. J Biol Chem. 1970 Apr 10;245(7):1537–1544. [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- French S. W. Fragility of liver mitochondria in ethanol-fed rats. Gastroenterology. 1968 Jun;54(6):1106–1114. [PubMed] [Google Scholar]
- French S. W., Ihrig T. J., Shaw G. P., Tanaka T. T., Norum M. L. The effect of ethanol on the fatty acid composition of hepatic microsomes and inner and outer mitochondrial membranes. Res Commun Chem Pathol Pharmacol. 1971 Jul-Sep;2(4):567–585. [PubMed] [Google Scholar]
- French S. W., Todoroff T. Hepatic mitochondrial fragility and permeability. Effect of ethanol and choline deficiency. Arch Pathol. 1970 Apr;89(4):329–336. [PubMed] [Google Scholar]
- Gains N., Dawson A. P. A kinetic analysis of the changes in fluorescence on the interaction of 8-anilinonaphthalene-1-sulphonate with submitochondrial particles. Biochem J. 1976 Aug 15;158(2):295–305. doi: 10.1042/bj1580295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R. Mitochondrial functions in an ethanol-induced fatty liver. J Biol Chem. 1973 Dec 10;248(23):8271–8280. [PubMed] [Google Scholar]
- Hosein E. A., Hofmann I., Linder E. The influence of chronic ethanol feeding to rats on the integrity of liver mitochondrial membrane as assessed with the Mg2+-stimulated ATPase enzyme. Arch Biochem Biophys. 1977 Sep;183(1):64–72. doi: 10.1016/0003-9861(77)90419-2. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., Struijk C. B., van der Beek A. Decrease in rate of ATP synthesis of isolated rat heart mitochondria induced by dietary erucic acid. Biochim Biophys Acta. 1970 Dec 15;218(3):564–566. doi: 10.1016/0005-2760(70)90025-1. [DOI] [PubMed] [Google Scholar]
- Janki R. M., Aithal H. N., McMurray W. C., Tustanoff E. R. The effect of altered membrane-lipid composition on enzyme activities of outer and inner mitochondrial membranes of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1078–1085. doi: 10.1016/s0006-291x(74)80298-6. [DOI] [PubMed] [Google Scholar]
- LIEBER C. S., JONES D. P., DECARLI L. M. EFFECTS OF PROLONGED ETHANOL INTAKE: PRODUCTION OF FATTY LIVER DESPITE ADEQUATE DIETS. J Clin Invest. 1965 Jun;44:1009–1021. doi: 10.1172/JCI105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L., Rubin E. Sequential production of fatty liver, hepatitis, and cirrhosis in sub-human primates fed ethanol with adequate diets. Proc Natl Acad Sci U S A. 1975 Feb;72(2):437–441. doi: 10.1073/pnas.72.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman B. J. Surface distribution of the fatty acid side chains of phosphatidylethanolamine in mixed phospholipid vesicles. Biochim Biophys Acta. 1975 Dec 1;413(2):157–162. doi: 10.1016/0005-2736(75)90100-5. [DOI] [PubMed] [Google Scholar]
- Mendenhall C. L., Bradford R. H., Furman R. H. Effect of ethanol on glycerolipid metabolism in rat liver. Biochim Biophys Acta. 1969 Dec 17;187(4):501–509. doi: 10.1016/0005-2760(69)90047-2. [DOI] [PubMed] [Google Scholar]
- Miceli J. N., Ferrell W. J. Effects of ethanol on membrane lipids 3. Quantitative changes in lipid and fatty acid composition of nonpolar and polar lipids of mouse total liver, mitochondria and microsomes following ethanol feeding. Lipids. 1973 Dec;8(12):722–727. doi: 10.1007/BF02531839. [DOI] [PubMed] [Google Scholar]
- Ozelkök S. I., Romani R. J. Restoration of energy-linked functions in "aging" rat-liver mitochondria. Life Sci. 1974 Apr 16;14(8):1427–1431. doi: 10.1016/0024-3205(74)90152-0. [DOI] [PubMed] [Google Scholar]
- Parce J. W., Cunningham C. C., Waite M. Mitochondrial phospholipase A2 activity and mitochondrial aging. Biochemistry. 1978 May 2;17(9):1634–1639. doi: 10.1021/bi00602a009. [DOI] [PubMed] [Google Scholar]
- Porta E. A., Hartroft W. S., De la Iglesia F. A. Hepatic changes associated with chronic alcoholism in rats. Lab Invest. 1965 Aug;14(8):1437–1455. [PubMed] [Google Scholar]
- Scarpa A., Lindsay J. G. Maintenance of energy-linked functions in rat-liver mitochondria aged in the presence of nupercaine. Eur J Biochem. 1972 Jun 9;27(3):401–407. doi: 10.1111/j.1432-1033.1972.tb01851.x. [DOI] [PubMed] [Google Scholar]
- Siliprandi D., Siliprandi N., Scutari G., Zoccarato F. Restoration of some energy linked processes lost during the ageing of rat liver mitochondria. Biochem Biophys Res Commun. 1973 Dec 10;55(3):563–567. doi: 10.1016/0006-291x(73)91180-7. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Reitz R. C. Studies of the acute and chronic effects of ethanol ingestion on choline oxidation. Ann N Y Acad Sci. 1976;273:194–204. doi: 10.1111/j.1749-6632.1976.tb52882.x. [DOI] [PubMed] [Google Scholar]
- Williams W. P., Layton D. G., Johnston C. An analysis of the binding of fluorescence probes in mitochondrial systems. J Membr Biol. 1977 May 6;33(1-2):21–40. doi: 10.1007/BF01869510. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]


