Abstract
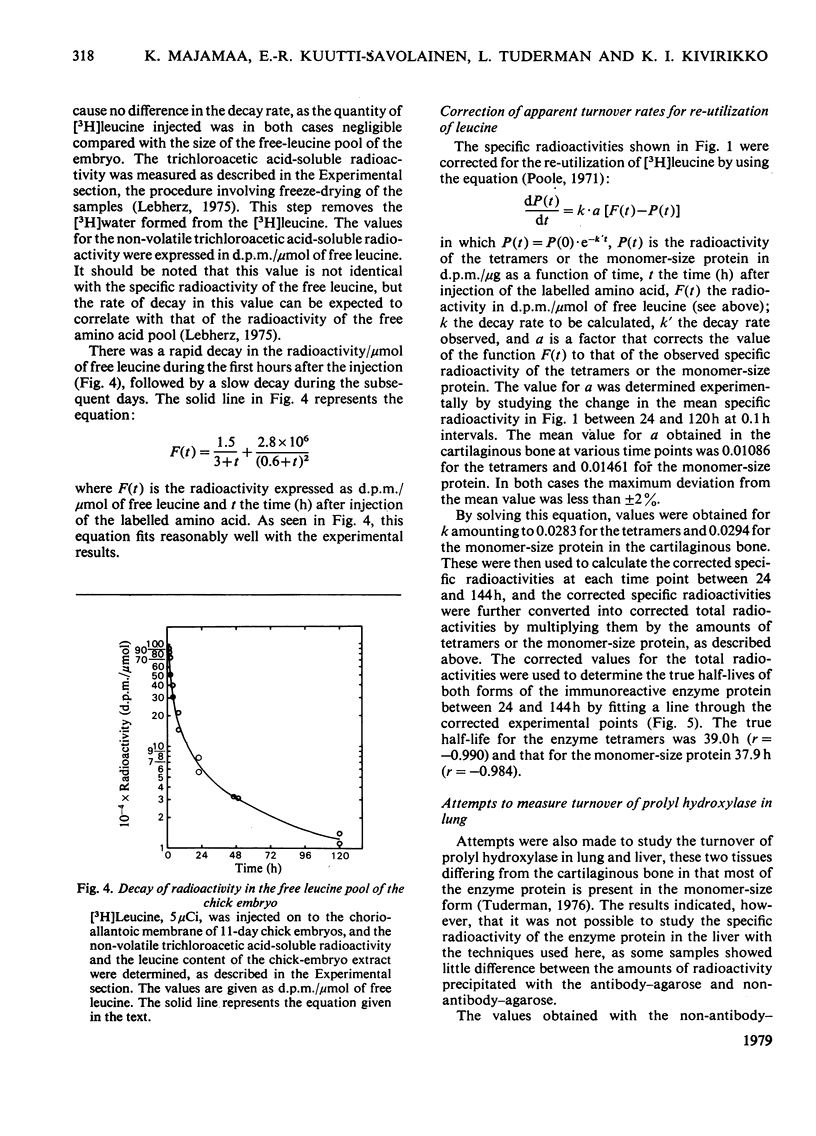
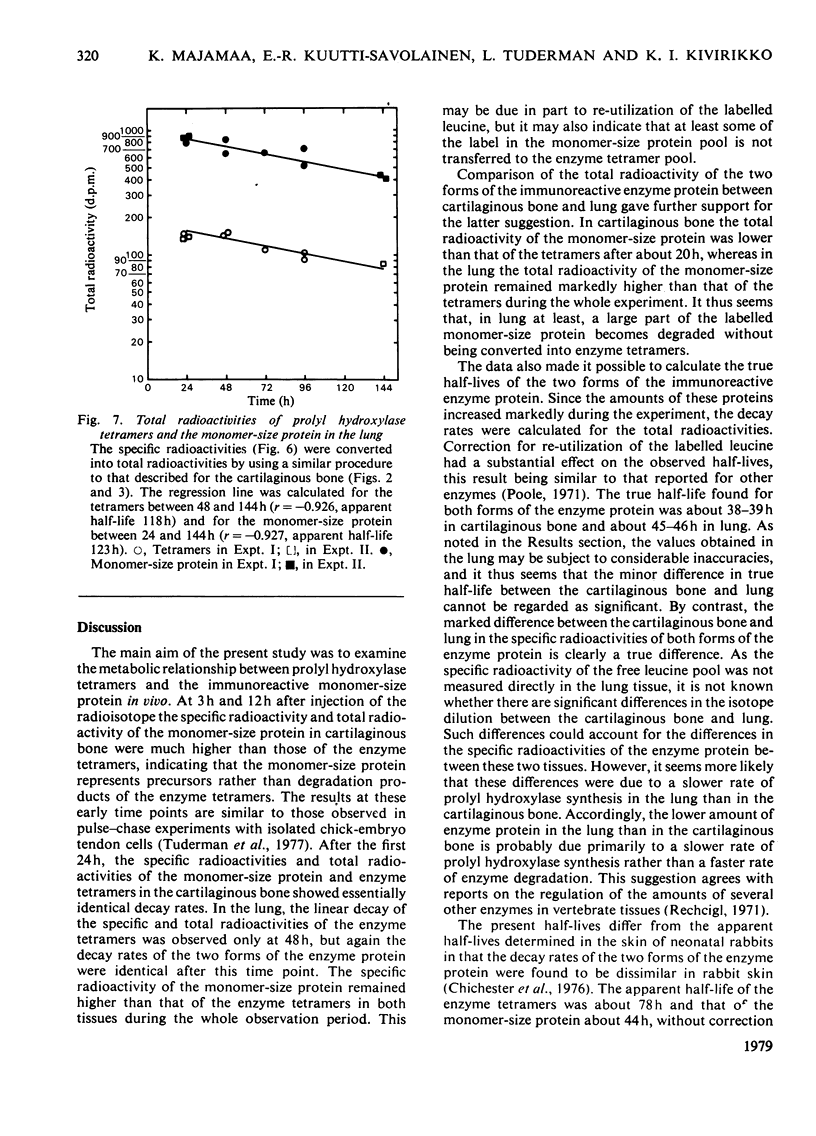
The turnover of prolyl hydroxylase and an immunoreactive protein that corresponds in size to the smaller subunit of the enzyme was studied in vivo after injection of [3H]leucine into 11-day chick embryos. The specific radioactivity and total radioactivity of the monomer-size protein were much higher than those of the enzyme tetramers in the cartilaginous bone at 3h and 12h after the radioisotope injection, indicating that the monomer-size protein represents precursors rather than degradation products of the enzyme tetramers. Between 24 and 144h after the injection the specific radioactivity and total radioactivity of the two forms of the enzyme protein showed essentially identical decay rates, the observed specific radioactivity of the monomer-size protein being about 120–130% and total radioactivity about 80% of that of the enzyme tetramers. The true half-life, when corrected for dilution caused by tissue growth and re-utilization of the [3H]leucine, was 37.9h for the monomer-size protein and 39.0h for the tetramers. The results obtained in the lung were less reliable owing to high blank radioactivity values in the immunoprecipitation, but even so some definite differences were found between this tissue and the cartilaginous bone. The specific radioactivity of both forms of the enzyme protein at 24h was only about 20–25% of that in the cartilaginous bone. The total radioactivity of the monomer-size protein in the lung remained about 5 times that of the enzyme tetramers, whereas it was only about 0.8 times that of the tetramers in the cartilaginous bone. As in the cartilaginous bone, the decay rates of both forms of the enzyme protein were essentially identical in the lung, with a true half-life of about 46h. The results suggest that the rate of prolyl hydroxylase synthesis is slower in the lung than in the cartilaginous bone, whereas the degradation rates are fairly similar in these two tissues. The data further suggest that, in the lung at least, a large part of the monomer-size protein became degraded without being converted into enzyme tetramers.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Cardinale G. J., Udenfriend S. Homology between a prolyl hydroxylase subunit and a tissue protein that crossreacts immunologically with the enzyme. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4420–4424. doi: 10.1073/pnas.74.10.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester C. O., 3rd, Fuller G. C., Cardinale G. J. In vivo labeling and turnover of prolyl hydroxylase and a related immunoreactive protein. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1056–1062. doi: 10.1016/0006-291x(76)90230-8. [DOI] [PubMed] [Google Scholar]
- Fuller G. C., Matoney A. L., Fisher D. O., Fausto N., Cardinale G. J. Increased collagen synthesis and the kinetic characteristics of prolyl hydroxylase in tissues of rabbits with experimental arteriosclerosis. Atherosclerosis. 1976 Sep;24(3):483–490. doi: 10.1016/0021-9150(76)90140-4. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Ascorbate increases the synthesis of procollagen hydroxyproline by cultured fibroblasts from chick embryo tendons without activation of prolyl hydroxyla. Biochim Biophys Acta. 1975 Dec 5;411(2):202–215. doi: 10.1016/0304-4165(75)90300-1. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Risteli L. Biosynthesis of collagen and its alterations in pathological states. Med Biol. 1976 Jun;54(3):159–186. [PubMed] [Google Scholar]
- Kuutti E. R., Tuderman L., Kivirikko K. I. Human prolyl hydroxylase. Purification, partial characterization and preparation of antiserum to the enzyme. Eur J Biochem. 1975 Sep 1;57(1):181–188. doi: 10.1111/j.1432-1033.1975.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G. Synthesis and degradation of fructose diphosphate aldolase isoenzymes in avian brain. J Biol Chem. 1975 Aug 10;250(15):5967–5975. [PubMed] [Google Scholar]
- McGee J. O., Udenfriend S. Partial purification and characterization of peptidyl proline hydroxylase precursor from mouse fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):216–221. doi: 10.1016/0003-9861(72)90209-3. [DOI] [PubMed] [Google Scholar]
- Poole B. The kinetics of disappearance of labeled leucine from the free leucine pool of rat liver and its effect on the apparent turnover of catalase and other hepatic proteins. J Biol Chem. 1971 Nov;246(21):6587–6591. [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Purification of rat prolyl hydroxylase and comparison of changes in prolyl hydroxylase activity with changes in immunoreactive prolyl hydroxylase. Biochem J. 1976 Aug 15;158(2):369–376. doi: 10.1042/bj1580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Tryggvason K., Kivirikko K. I. Effect of hepatic injury on prolyl 3-hydroxylase and 4-hydroxylase activities in rat liver and on immunoreactive prolyl 4-hydroxylase concentrations in the liver and serum. Biochem J. 1978 Jan 15;170(1):129–135. doi: 10.1042/bj1700129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., McGee J. O., Udenfriend S. Prolyl hydroxylase and an immunologically related protein in mammalian tissues. Arch Biochem Biophys. 1974 Jan;160(1):340–345. doi: 10.1016/s0003-9861(74)80042-1. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., Udenfriend S. Activation of prolyl hydroxylase in L-929 fibroblasts by ascorbic acid. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1090–1093. doi: 10.1073/pnas.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuderman L. Developmental changes in prolyl hydroxylase activity and protein in chick embryo. Eur J Biochem. 1976 Jul 15;66(3):615–621. doi: 10.1111/j.1432-1033.1976.tb10589.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kivirikko K. I. Immunoreactive prolyl hydroxylase in human skin, serum and synovial fluid: changes in the content and components with age. Eur J Clin Invest. 1977 Aug;7(4):295–299. doi: 10.1111/j.1365-2362.1977.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. Radiommunoassay for human and chick prolyl hydroxylases. Eur J Biochem. 1975 Dec 15;60(2):399–405. doi: 10.1111/j.1432-1033.1975.tb21016.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Oikarinen A., Kivirikko K. I. Tetramers and monomers of prolyl hydroxylase in isolated chick-embryo tendon cells. The association of inactive monomers to active tetramers and a preliminary characterization of the intracellular monomer-size protein. Eur J Biochem. 1977 Sep;78(2):547–556. doi: 10.1111/j.1432-1033.1977.tb11768.x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi T. M., Puistola U., Anttinen H., Kivirikko K. I. Affinity chromatography of lysyl hydroxylase on concanavalin A-agarose. Biochim Biophys Acta. 1977 Jul 8;483(1):215–219. doi: 10.1016/0005-2744(77)90024-9. [DOI] [PubMed] [Google Scholar]


