Abstract
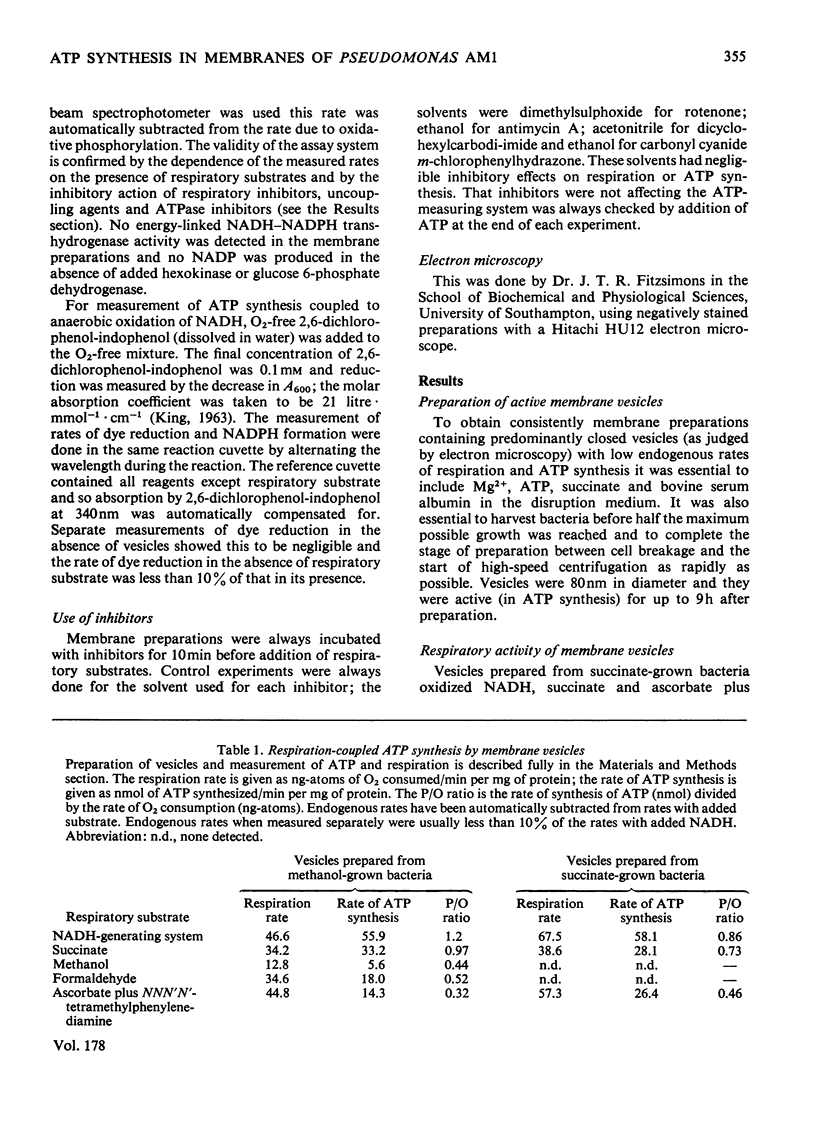
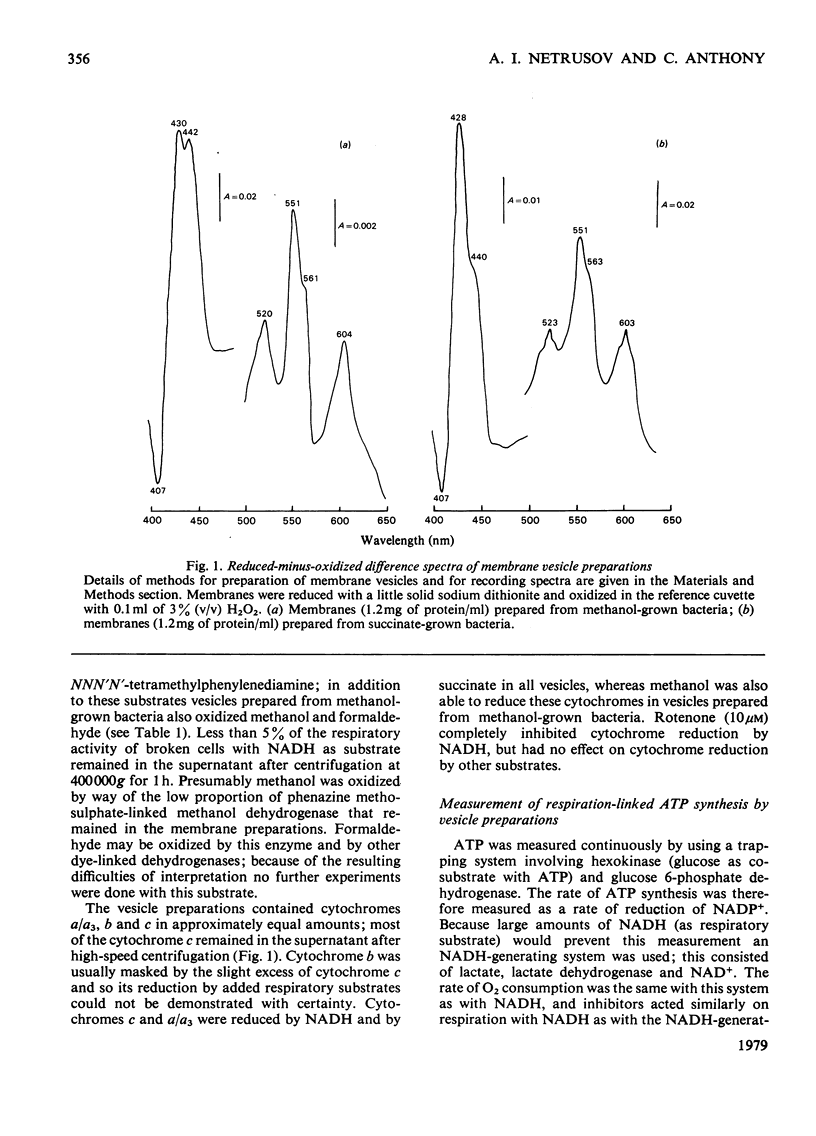
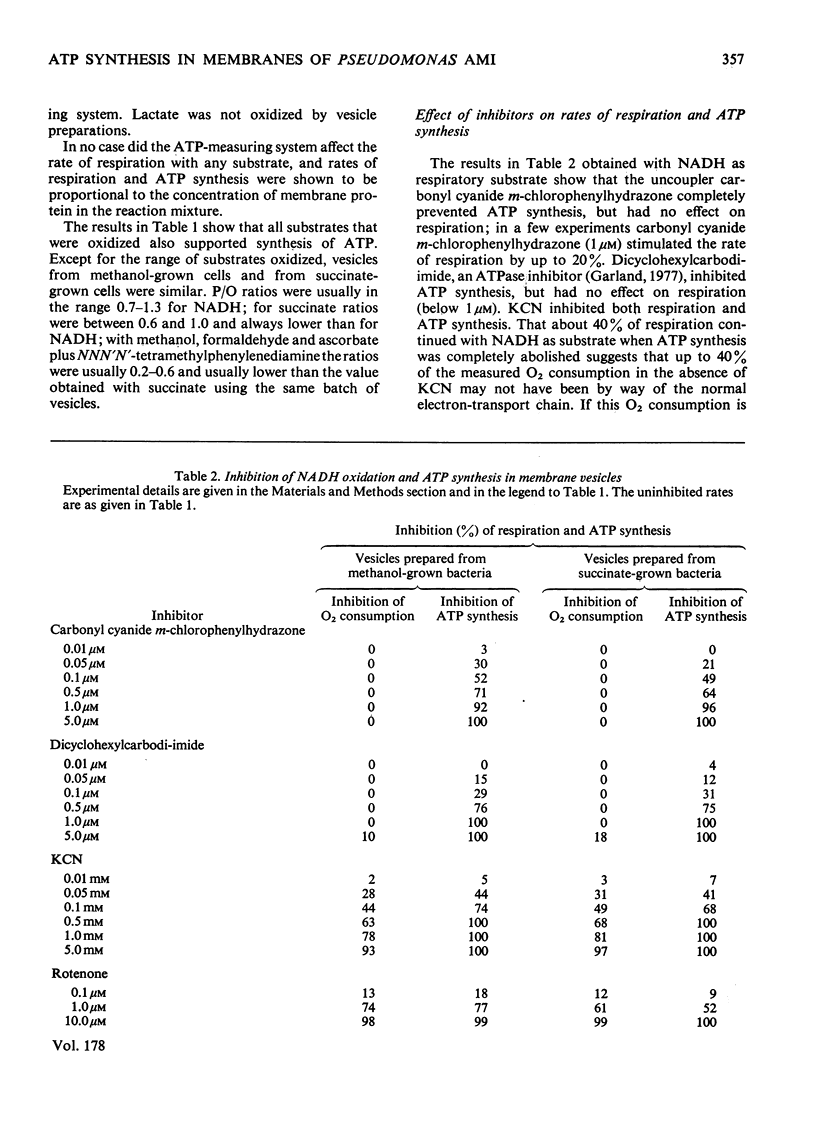
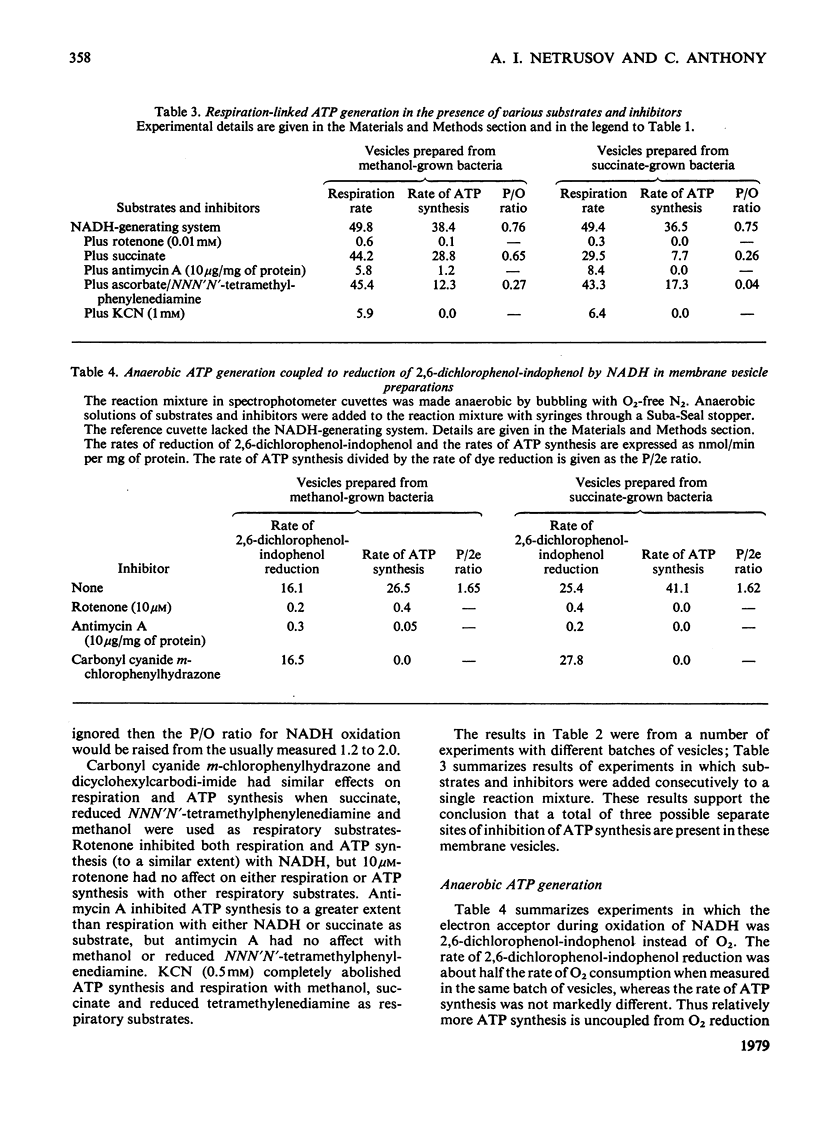
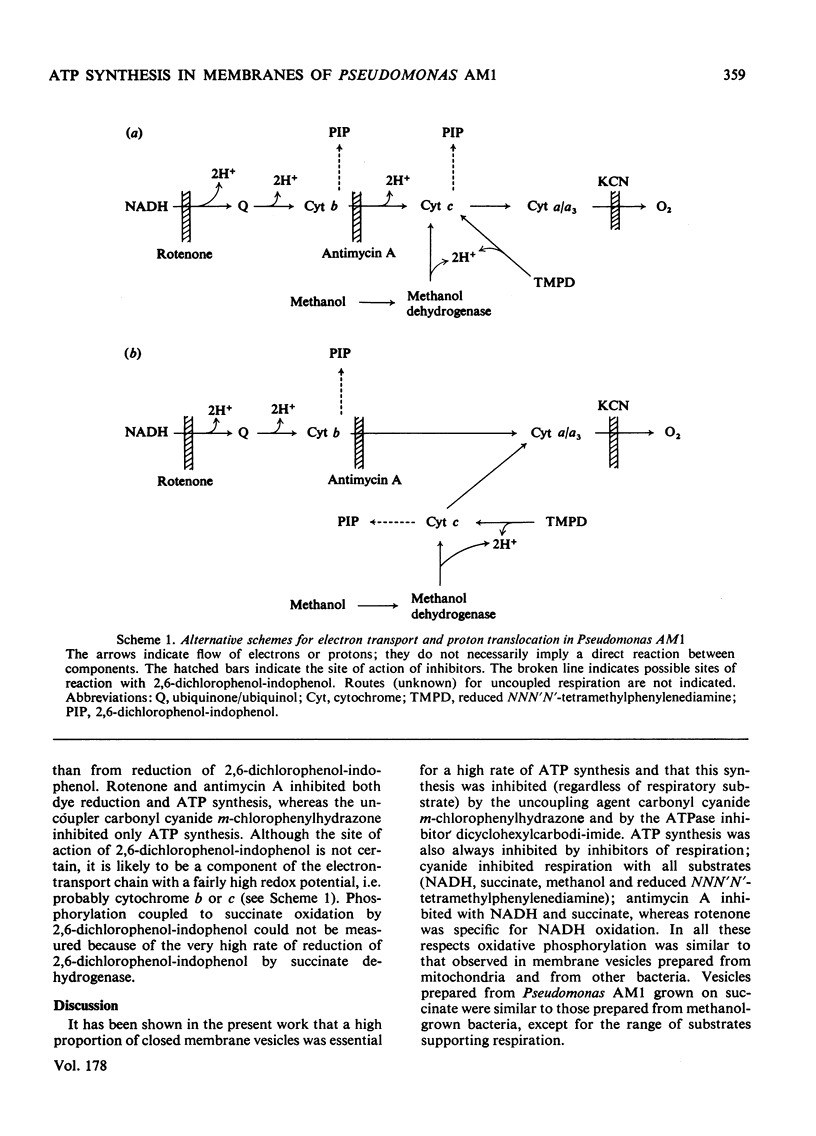
A method is described for preparation of membrane vesicles (diameter 80nm) capable of respiration-linked ATP synthesis. Vesicles prepared from succinate-grown bacteria oxidized NADH, succinate and ascorbate plus NNN′N′-tetramethylphenylenediamine; vesicles prepared from methanol-grown bacteria also oxidized methanol and formaldehyde, but they were otherwise identical. The uncoupling agent carbonyl cyanide chlorophenylhydrazone and the adenosine triphosphatase inhibitor dicyclohexylcarbodi-imide both inhibited ATP synthesis, whereas they had no effect on the rate of respiration. Rotenone inhibited ATP synthesis and respiration with NADH as substrate; antimycin A inhibited with succinate as substrate, and cyanide inhibited with all substrates. P/O ratios were usually 0.7–1.3 with NADH, 0.6–1.0 with succinate and 0.2–0.6 with reduced NNN′N′-tetramethylphenylenediamine or methanol as respiratory substrate. When 2,6-dichlorophenol-indophenol was used as an alternative electron acceptor to O2 (NADH as donor) the P/2e ratio was 1.65. Although these P/O ratios are minimum values, because they do not take into account unknown amounts of uncoupled O2 consumption, they are consistent with previous proposals [O'Keeffe & Anthony (1978) Biochem, J. 170, 561–567] based on measurements of proton translocation in whole cells. The results also confirm that methanol dehydrogenase and cytochromes c and a/a3 are arranged so that the first step in methanol oxidation is coupled to synthesis of ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The microbial metabolism of C1 compounds. The cytochromes of Pseudomaonas AM1. Biochem J. 1975 Feb;146(2):289–298. doi: 10.1042/bj1460289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J. 1967 Sep;104(3):960–969. doi: 10.1042/bj1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maclennan D. G., Ousby J. C., Vasey R. B., Cotton N. T. The influence of dissolved oxygen on Pseudomonas AM1 grown on methanol in continuous culture. J Gen Microbiol. 1971 Dec;69(3):395–404. doi: 10.1099/00221287-69-3-395. [DOI] [PubMed] [Google Scholar]
- Netrusov A. I., Rodionov Y. V., Kondratieva E. N. ATP-generation coupled with C1-compound oxidation by methylotrophic bacterium Pseudomonas sp.2. FEBS Lett. 1977 Apr 1;76(1):56–58. doi: 10.1016/0014-5793(77)80119-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe D. T., Anthony C. The microbial metabolism of Cl compounds. The stoicheiometry of respiration-driven proton translocation in Pseudomonas AM1 and in a mutant lacking cytochrome c. Biochem J. 1978 Mar 15;170(3):561–567. doi: 10.1042/bj1700561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson D., Anthony C. The microbial metabolism of C1 compounds. The electron-transport chain of Pseudomonas am1. Biochem J. 1975 Nov;152(2):349–356. doi: 10.1042/bj1520349. [DOI] [PMC free article] [PubMed] [Google Scholar]


