Abstract
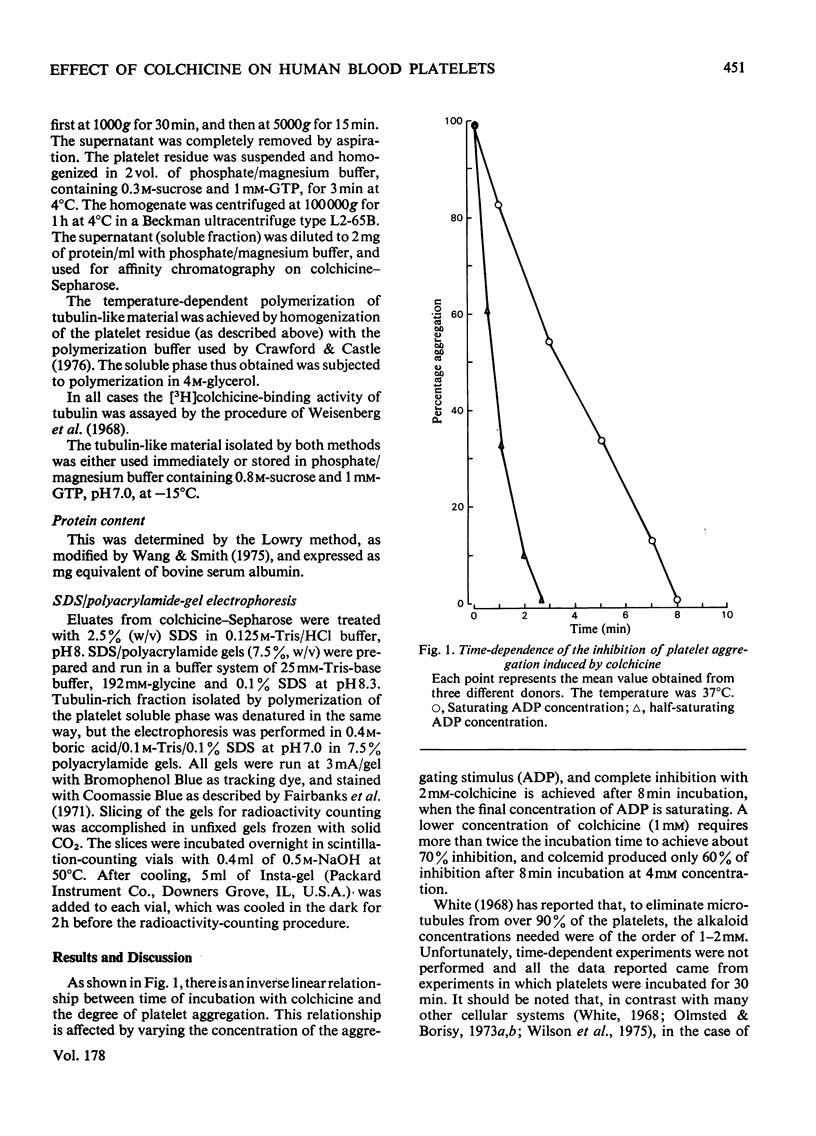
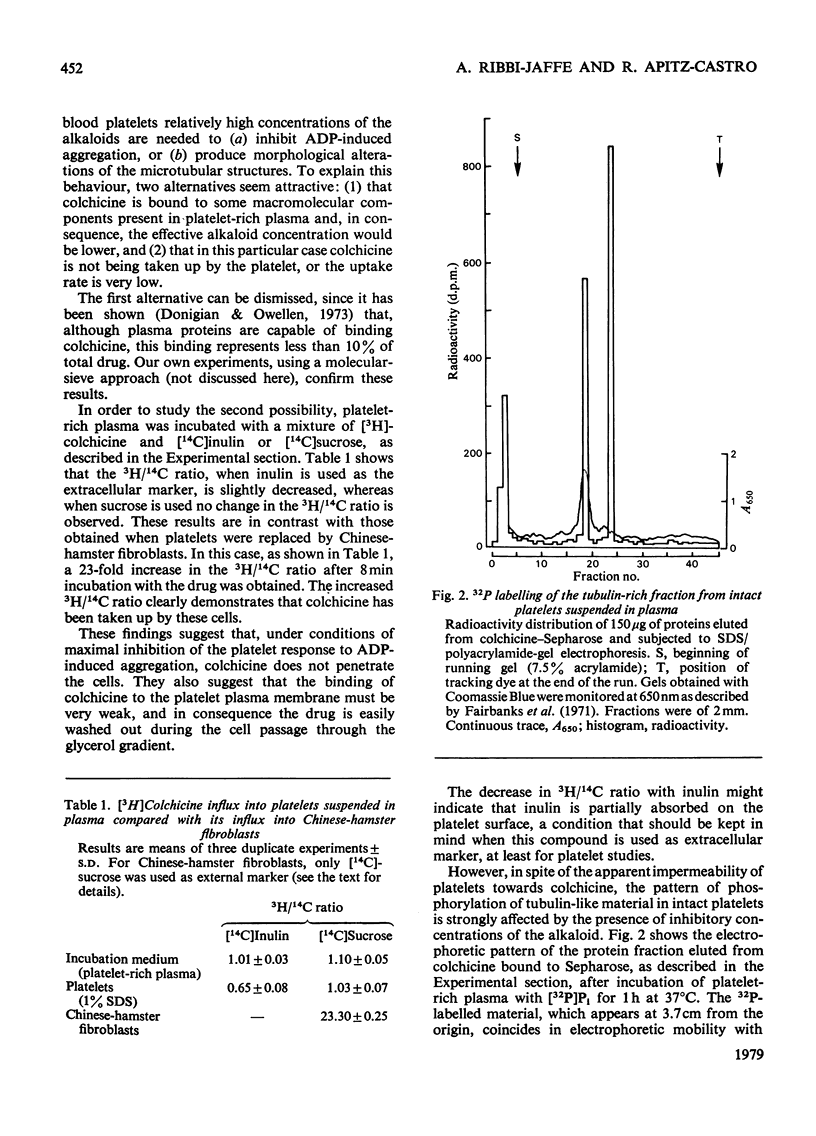
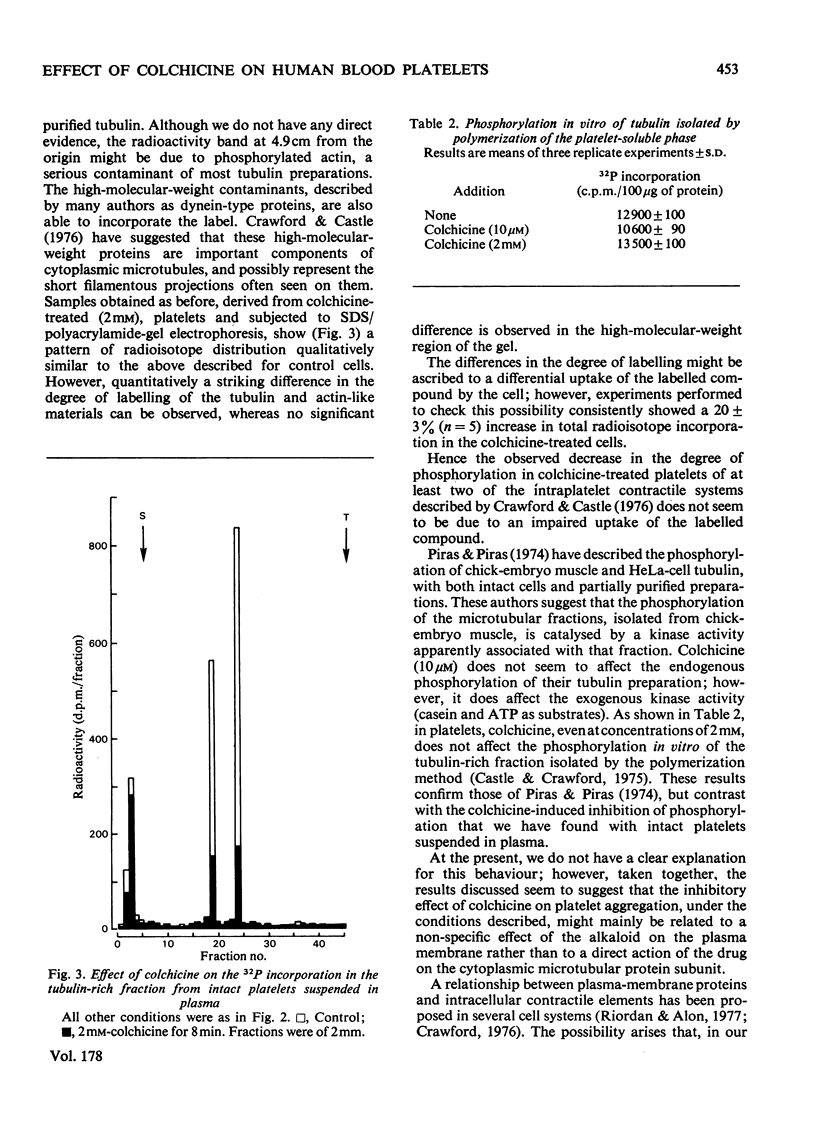
The effects of colchicine on ADP-induced aggregation and on the phosphorylation of tubulin-like protein from human blood platelets were studied. Colchicine at 2mM concentration completely inhibits ADP-induced aggregation after 8min incubation. Under the same inhibitory conditions, phosphorylation of tubulin-like materials in intact platelets was also impaired whereas the endogenous kinase activity of tubulin, isolated through polymerization--depolymerization cycles, was not affected. It was also shown that, under conditions of maximal inhibition of both aggregation and tubulin phosphorylation, colchicine does not penetrate into the cells. The results obtained suggest that the effect of colchicine on platelet aggregation might be mainly, although not exclusively, due to a non-specific effect of the alkaloid on the plasma membrane, rather than to a direct action of the drug on the microtubular protein subunits.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apitz-Castro R., Ramírez E., Maingon R., de Murciano A., Ribbi A. Plasma membrane phosphorylation by endogenous phosphate donors in human blood platelets. Selectivity of the action of dibutyryl cyclic AMP. Biochim Biophys Acta. 1976 Dec 2;455(2):371–382. doi: 10.1016/0005-2736(76)90312-6. [DOI] [PubMed] [Google Scholar]
- Barber A. J., Jamieson G. A. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970 Dec 10;245(23):6357–6365. [PubMed] [Google Scholar]
- Castle A. G., Crawford N. Isolation of tubulin from pig platelets. FEBS Lett. 1975 Mar 1;51(1):195–200. doi: 10.1016/0014-5793(75)80886-6. [DOI] [PubMed] [Google Scholar]
- Castle A. G., Crawford N. The isolation and characterisation of platelet microtubule proteins. Biochim Biophys Acta. 1977 Sep 27;494(1):76–91. doi: 10.1016/0005-2795(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Donigian D. W., Owellen R. J. Interaction of vinblastine, vincristine and colchicine with serum proteins. Biochem Pharmacol. 1973 Sep 1;22(17):2113–2119. doi: 10.1016/0006-2952(73)90110-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Lüscher E. F., Bettex-Galland M. Thrombosthenin, the contractile protein of blood platelets. New facts and problems. Pathol Biol (Paris) 1972 Dec;(Suppl):89–101. [PubMed] [Google Scholar]
- Margulis L. Colchicine-sensitive microtubules. Int Rev Cytol. 1973;34:333–361. doi: 10.1016/s0074-7696(08)61939-7. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Piras M. M., Piras R. Phosphorylation of vinblastine-isolated microtubules from chick-embryonic muscles. Eur J Biochem. 1974 Sep 16;47(3):443–452. doi: 10.1111/j.1432-1033.1974.tb03711.x. [DOI] [PubMed] [Google Scholar]
- Rieber M., Bacalao J. Selective enhancement of cytoplasmic protein species and continued histone synthesis during inhibition of DNA synthesis in temperature-sensitive Chinese hamster cells. Cancer Res. 1974 Nov;34(11):3083–3088. [PubMed] [Google Scholar]
- Riordan J. R., Alon N. Binding of [3H]ctyochalasin B and [3H]colchicine to isolated liver plasma membranes. Biochim Biophys Acta. 1977 Feb 4;464(3):547–561. doi: 10.1016/0005-2736(77)90029-3. [DOI] [PubMed] [Google Scholar]
- Schmitt H., Littauer U. Z. Affinity chromatography of tubulin. Methods Enzymol. 1974;34:623–627. doi: 10.1016/s0076-6879(74)34084-0. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- White J. G. Effects of colchicine and Vinca alkaloids on human platelets. I. Influence on platelet microtubules and contractile function. Am J Pathol. 1968 Aug;53(2):281–291. [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]


