Abstract
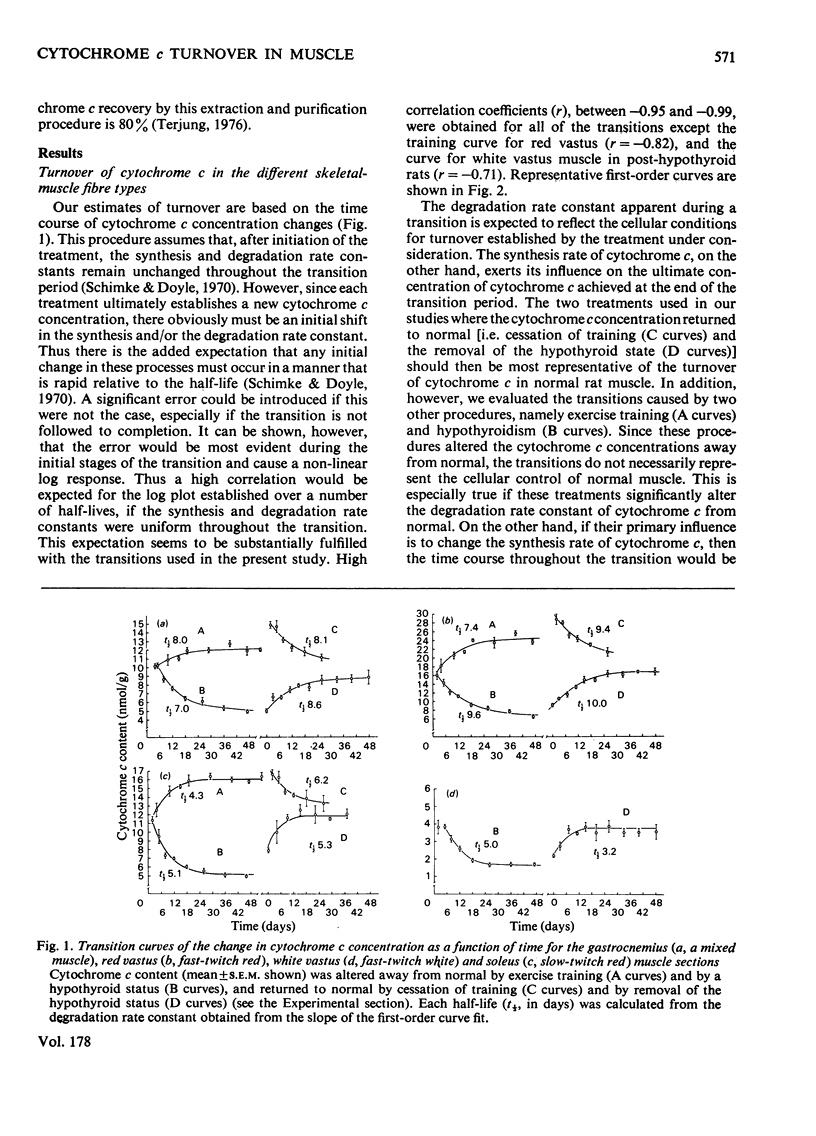
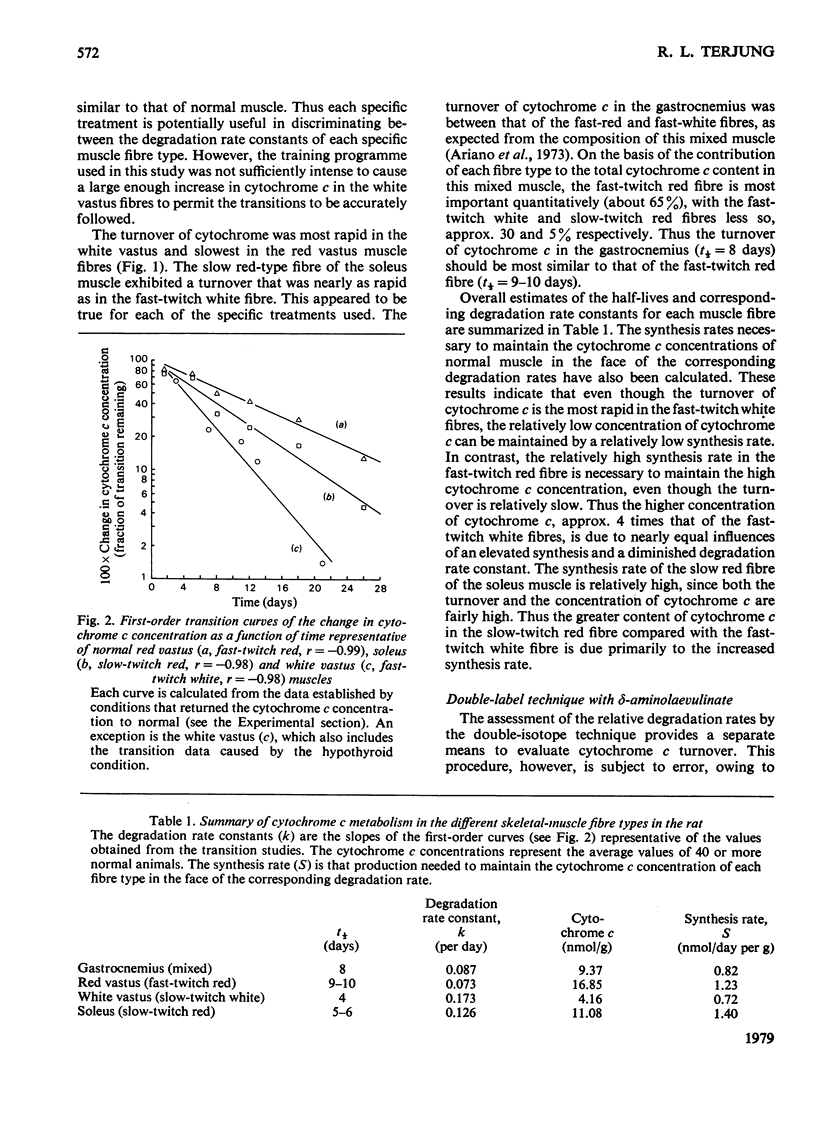
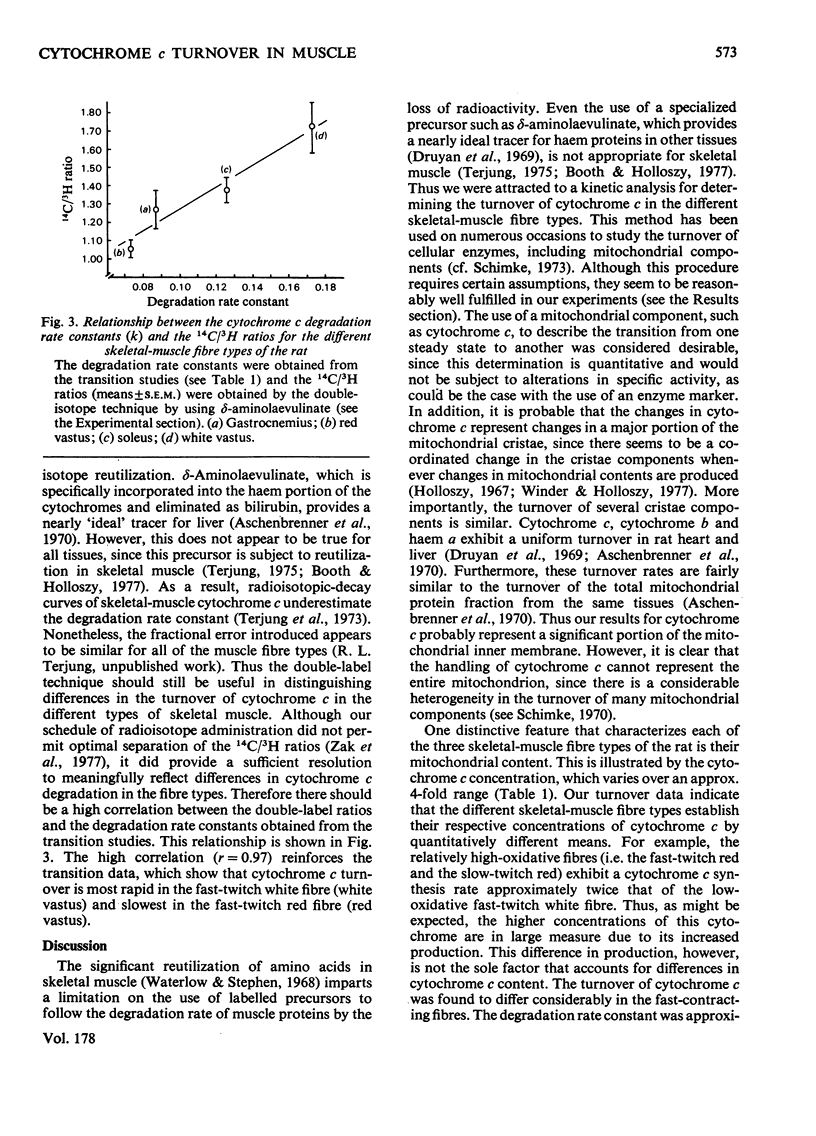
The turnover of cytochrome c was determined in the three skeletal-muscle fibre types of adult male rats by a kinetic analysis that followed the time course of cytochrome c content change. Confirming evidence was obtained with double-labelling studies using delta-aminolaevulinate. Cytochrome c turnover was most rapid in the low-oxidative fast-twitch white fibre [t1/2 (half-life) about 4 days], slowest in the high-oxidative fast-twitch red fibre (t1/2 9-10 days) and relatively rapid in the high-oxidative slow-twitch red fibre (t1/2 5-6 days). Thus cytochrome c turnover does not strictly conform to either the appearance (i.e. red or white) or the contractile characteristics (i.e. fast or slow) of the muscle fibres. The synthesis rates needed to maintain the corresponding cytochrome c concentrations, however, were similarly high in the two mitochondria-rich red fibre types. These data illustrate that both the synthesis and degradation processes are important in establishing the cytochrome c concentrations that distinguish the different skeletal-muscle fibre types.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner B., Druyan R., Albin R., Rabinowitz M. Haem a, cytochrome c and total protein turnover in mitochondria from rat heart and liver. Biochem J. 1970 Sep;119(2):157–160. doi: 10.1042/bj1190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K. M., Hooker A. M., Campbell P. J., Lewis R. E. Enzyme changes in neonatal skeletal muscle: effect of thyroid deficiency. Am J Physiol. 1978 Sep;235(3):C97–102. doi: 10.1152/ajpcell.1978.235.3.C97. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Winder W. W., Terjung R. L., Holloszy J. O. Glycolytic enzymes in different types of skeletal muscle: adaptation to exercise. Am J Physiol. 1973 Oct;225(4):962–966. doi: 10.1152/ajplegacy.1973.225.4.962. [DOI] [PubMed] [Google Scholar]
- Booth F. W., Holloszy J. O. Cytochrome c turnover in rat skeletal muscles. J Biol Chem. 1977 Jan 25;252(2):416–419. [PubMed] [Google Scholar]
- Booth F. W., Holloszy J. O. Effect of thyroid hormone administration on synthesis and degradation of cytochrome c in rat liver. Arch Biochem Biophys. 1975 Apr;167(2):674–677. doi: 10.1016/0003-9861(75)90511-1. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Druyan R., DeBernard B., Rabinowitz M. Turnover of cytochromes labeled with delta-aminolevulinic acid-3H in rat liver. J Biol Chem. 1969 Nov 10;244(21):5874–5878. [PubMed] [Google Scholar]
- Fitts R. H., Booth F. W., Winder W. W., Holloszy J. O. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol. 1975 Apr;228(4):1029–1033. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- Gross N. J. Control of mitochondrial turnover under the influence of thyroid hormone. J Cell Biol. 1971 Jan;48(1):29–40. doi: 10.1083/jcb.48.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J. O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967 May 10;242(9):2278–2282. [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- IINO S., YAMADA T., GREER M. A. Effect of graded doses of propylthiouracil on biosynthesis of thyroid hormones. Endocrinology. 1961 Apr;68:582–588. doi: 10.1210/endo-68-4-582. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Control of enzyme levels in mammalian tissues. Adv Enzymol Relat Areas Mol Biol. 1973;37:135–187. doi: 10.1002/9780470122822.ch3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Terjung R. L. Cytochrome c turnover in skeletal muscle. Biochem Biophys Res Commun. 1975 Sep 2;66(1):173–178. doi: 10.1016/s0006-291x(75)80310-x. [DOI] [PubMed] [Google Scholar]
- Terjung R. L., Koerner J. E. Biochemical adaptations in skeletal muscle of trained thyroidectomized rats. Am J Physiol. 1976 May;230(5):1194–1197. doi: 10.1152/ajplegacy.1976.230.5.1194. [DOI] [PubMed] [Google Scholar]
- Terjung R. L. Muscle fiber involvement during training of different intensities and durations. Am J Physiol. 1976 Apr;230(4):946–950. doi: 10.1152/ajplegacy.1976.230.4.946. [DOI] [PubMed] [Google Scholar]
- Terjung R. L., Winder W. W., Baldwin K. M., Holloszy J. O. Effect of exercise on the turnover of cytochrome c in skeletal muscle. J Biol Chem. 1973 Nov 10;248(21):7404–7406. [PubMed] [Google Scholar]
- Tipton C. M., Terjung R. L., Barnard R. J. Response of thyroidectomized rats to training. Am J Physiol. 1968 Nov;215(5):1137–1142. doi: 10.1152/ajplegacy.1968.215.5.1137. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- Winder W. W., Baldwin K. M., Holloszy J. O. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974 Sep 16;47(3):461–467. doi: 10.1111/j.1432-1033.1974.tb03713.x. [DOI] [PubMed] [Google Scholar]
- Winder W. W., Holloszy J. O. Response of mitochondria of different types of skeletal muscle to thyrotoxicosis. Am J Physiol. 1977 May;232(5):C180–C184. doi: 10.1152/ajpcell.1977.232.5.C180. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Prior G., Rabinowitz M. Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem. 1977 May 25;252(10):3430–3435. [PubMed] [Google Scholar]


