Abstract
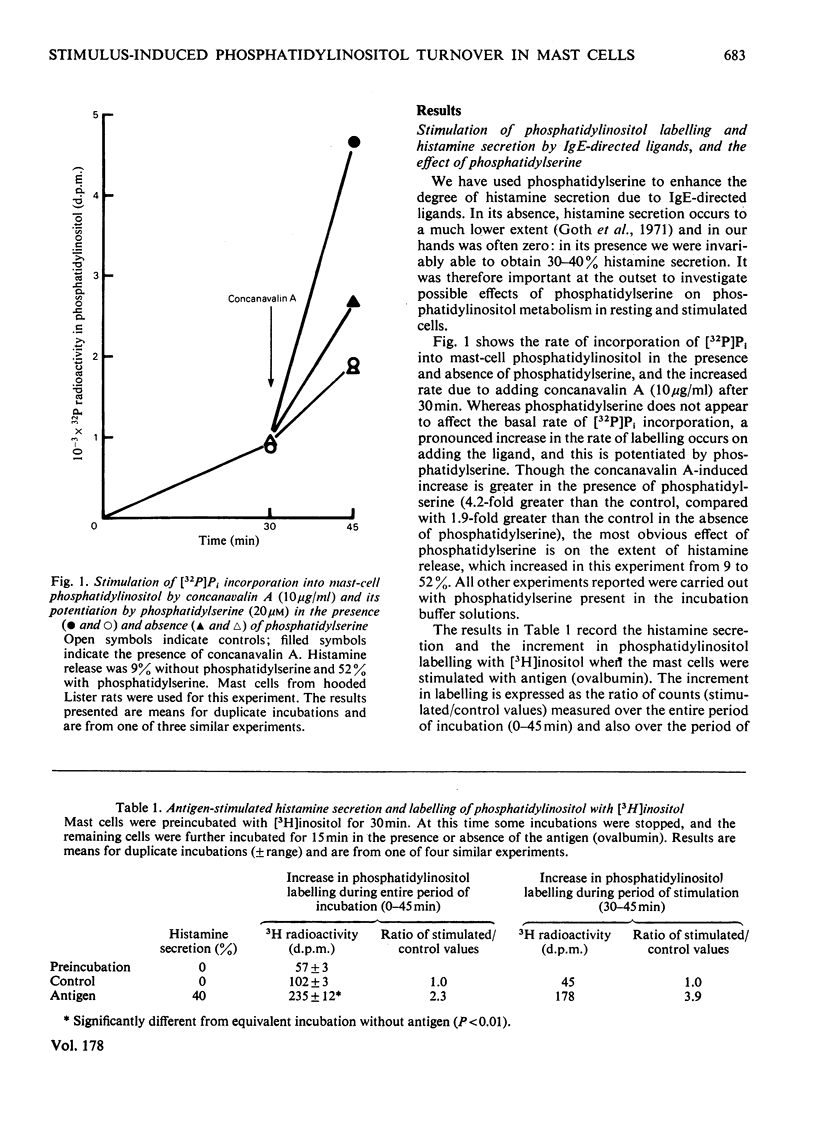
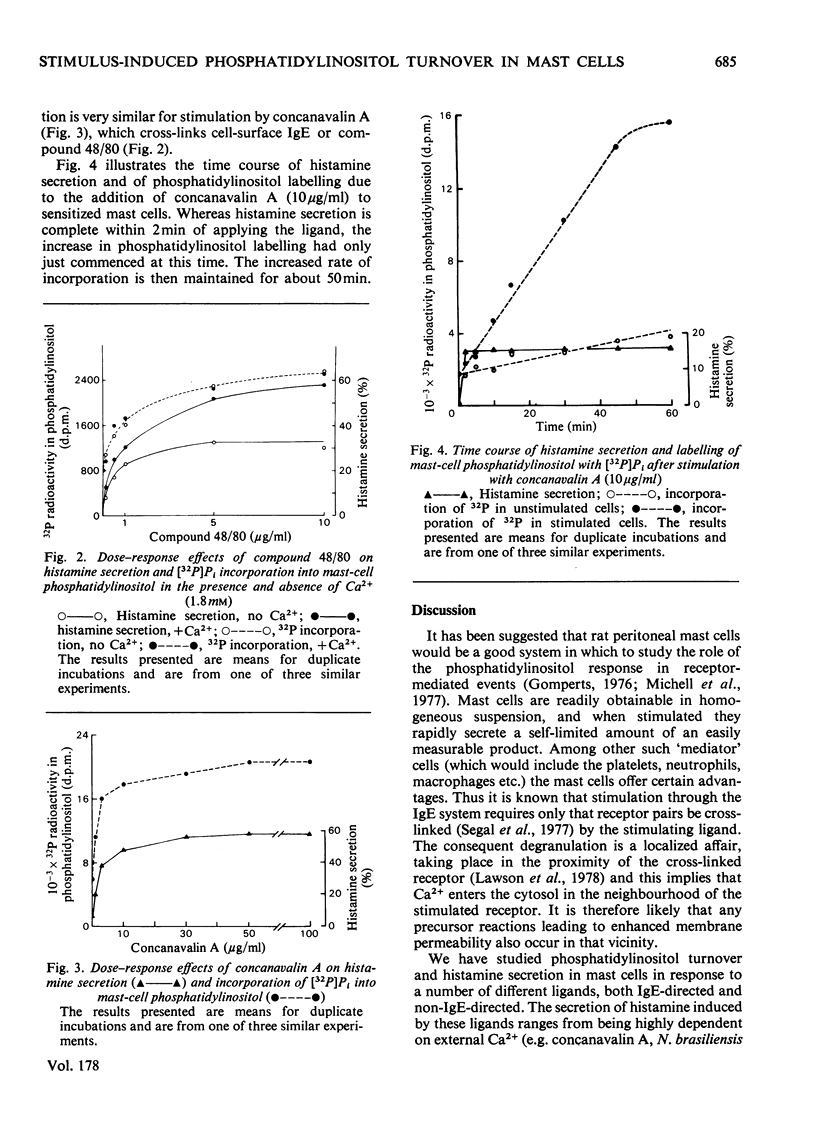
Histamine secretion and phosphatidylinositol turnover were compared in antigen-sensitized rat peritoneal mast cells stimulated with a number of different ligands. A small and variable increase in the incorporation of [32P]Pi and of [3H]inositol into phosphatidylinositol was observed when the cells were treated with immunoglobulin E-directed ligands (antigens and concanavalin A), and this was accompanied by a low amount of secretion (<10% of total cell histamine). In the presence of added phosphatidylserine, the addition of immunoglobulin E-directed ligands invariably led to an enhanced rate (approx. 4-fold) of labelling of phosphatidylinositol and, in the presence of Ca2+, this was accompanied by the secretion of histamine. The labelling of phosphatidylinositol and histamine secretion were also stimulated by chymotrypsin and compound 48/80. Whereas the phosphatidylinositol response did not require the presence of extracellular Ca2+, the secretion of histamine was either enhanced or dependent on extracellular Ca2+ (depending on the ligand used). The dependence on ligand concentration for the phosphatidylinositol response and histamine secretion were similar. The increased isotopic incorporation into phosphatidylinositol continued for about 1h although histamine secretion (elicited with concanavalin A) stopped within 2min. These results support the proposition that metabolic events involving phosphatidylinositol play a necessary intermediate role in the regulation of Ca2+ channels by ligand-activated receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. H., Adamik R. Differences in requirements and actions of various histamine-releasing agents. Biochem Pharmacol. 1978 Feb 15;27(4):497–503. doi: 10.1016/0006-2952(78)90384-2. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Michell R. H. Stimulation of the breakdown and resynthesis of of phosphatidylinositol in rat hepatocytes by angiotensin, vasopressin and adrenaline. Biochem Soc Trans. 1978;6(5):1033–1035. doi: 10.1042/bst0061033. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J., Hulme E. C. Biochemical studies on muscarinic acetylcholine receptors. J Neurochem. 1976 Jul;27(1):7–16. doi: 10.1111/j.1471-4159.1976.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Bloom G. D., Haegermark O. A study on morphological changes and histamine release induced by compound 48/80 in rat peritoneal mast cells. Exp Cell Res. 1965 Dec;40(3):637–654. doi: 10.1016/0014-4827(65)90241-7. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Stanworth D. R. Isolation of rat peritoneal mast cells in high yield and purity. Methods Cell Biol. 1976;14:365–378. doi: 10.1016/s0091-679x(08)60496-3. [DOI] [PubMed] [Google Scholar]
- DIAMANT B., UVNAS B. Evidence for energy-requiring processes in histamine release and mast cell degranulation in rat tissues induced by compound 48/80. Acta Physiol Scand. 1961 Nov-Dec;53:315–329. doi: 10.1111/j.1748-1716.1961.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between 5-hydroxytryptamine activation of phosphatidylinositol hydrolysis and calcium-ion entry in Calliphora salivary glands. Biochem Soc Trans. 1978;6(5):1038–1039. doi: 10.1042/bst0061038. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. M., Gomperts B. D. Quercetin: a novel inhibitor of Ca2+ influx and exocytosis in rat peritoneal mast cells. Biochim Biophys Acta. 1977 Aug 15;469(1):52–60. doi: 10.1016/0005-2736(77)90325-x. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Garland L. G. Desensitization in the process of histamine secretion induced by antigen and dextran. J Physiol. 1974 Jun;239(2):381–391. doi: 10.1113/jphysiol.1974.sp010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Garland L. G., Mongar J. L. Inhibition by cromoglycate of histamine release from rat peritoneal mast cells induced by mixtures of dextran, phosphatidyl serine and calcium ions. Br J Pharmacol. 1974 Jan;50(1):137–143. doi: 10.1111/j.1476-5381.1974.tb09601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., AUSTEN K. F., RAPP H. J. In vitro studies of reversed anaphylaxis with rat cells. Immunology. 1963 May;6:226–245. [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Suria A. The link between agonist action and response in smooth muscle. Annu Rev Pharmacol. 1971;11:303–326. doi: 10.1146/annurev.pa.11.040171.001511. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Immune mechanisms of reversed type reaginic hypersensitivity. J Immunol. 1969 Sep;103(3):588–595. [PubMed] [Google Scholar]
- Jafferji S. S., Michell R. H. Effects of calcium-antagonistic drugs on the stimulation by carbamoylcholine and histamine of phosphatidylinositol turnover in longitudinal smooth muscle of guinea-pig ileum. Biochem J. 1976 Nov 15;160(2):163–169. doi: 10.1042/bj1600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferji S. S., Michell R. H. Investigation of the relationship between cell-surface calcium-ion gating and phosphatidylinositol turnover by comparison of the effects of elevated extracellular potassium ion concentration on ileium smooth muscle and pancreas. Biochem J. 1976 Nov 15;160(2):397–399. doi: 10.1042/bj1600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferji S. S., Michell R. H. Muscarinic cholinergic stimulation of phosphatidylinositol turnover in the longitudinal smooth muscle of guinea-pig ileum. Biochem J. 1976 Mar 15;154(3):653–657. doi: 10.1042/bj1540653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Stewart D. C. The significance of circulating IgE. Correlation of amount of circulating reaginic antibody with cutaneous sensitivity in the rat. Immunology. 1973 Jan;24(1):37–45. [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Enhanced phosphatidylinositol breakdown as a calcium-indepepdnet response of rat parotid fragments to substance P. Biochem Soc Trans. 1978;6(5):1035–1037. doi: 10.1042/bst0061035. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. The relationship of calcium to receptor-controlled stimulation of phosphatidylinositol turnover. Effects of acetylcholine, adrenaline, calcium ions, cinchocaine and a bivalent cation ionophore on rat parotid-gland fragments. Biochem J. 1975 Jun;148(3):479–485. doi: 10.1042/bj1480479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagayama M., Douglas W. W. Electron microscope evidence of calcium-induced exocytosis in mast cells treated with 48-80 or the ionophores A-23187 and X-537A. J Cell Biol. 1974 Aug;62(2):519–526. doi: 10.1083/jcb.62.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Concanavalin A, a model "antigen" for the in vitro detection of cell-bound reaginic antibody in the rat. Clin Exp Immunol. 1973 Jan;13(1):139–147. [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Verrinder T. R., Hems D. A. The influence of extracellular calcium concentration on the vasopressin-stimulated incorporation of inorganic phosphate into phosphatidylinositol in hepatocyte suspensions. Biochem Soc Trans. 1978;6(5):1031–1033. doi: 10.1042/bst0061031. [DOI] [PubMed] [Google Scholar]
- Lagunoff D., Chi E. Y., Wan H. Effects of chymotrypsin and trypsin on rat peritoneal mast cells. Biochem Pharmacol. 1975 Sep 1;24(17):1573–1578. doi: 10.1016/0006-2952(75)90081-7. [DOI] [PubMed] [Google Scholar]
- Lawson D., Fewtrell C., Gomperts B., Raff M. Anti-immunoglobulin-induced histamine secretion by rat peritoneal mast cells studied by immunoferritin electron microscopy. J Exp Med. 1975 Aug 1;142(2):391–402. doi: 10.1084/jem.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D., Fewtrell C., Raff M. C. Localized mast cell degranulation induced by concanavalin A-sepharose beads. Implications for the Ca2+ hypothesis of stimulus-secretion coupling. J Cell Biol. 1978 Nov;79(2 Pt 1):394–400. doi: 10.1083/jcb.79.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTA I. Action of anaphylactic shock and anaphylatoxin on mast cells and histamine in rats. Br J Pharmacol Chemother. 1957 Dec;12(4):453–456. doi: 10.1111/j.1476-5381.1957.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro A. M., Bennich H. Concanavalin A induced histamine release from human basophils in vitro. Immunology. 1977 Jul;33(1):51–58. [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. Receptor occupancy dose--response curve suggests that phosphatidyl-inositol breakdown may be intrinsic to the mechanism of the muscarinic cholinergic receptor. FEBS Lett. 1976 Oct 15;69(1):1–5. doi: 10.1016/0014-5793(76)80640-0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jones L. M. Enhanced phosphatidylinositol labelling in rat parotid fragments exposed to alpha-adrenergic stimulation. Biochem J. 1974 Jan;138(1):47–52. doi: 10.1042/bj1380047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Jones L. M., Jafferji S. S. A possible role for phosphatidylinositol breakdown in muscarinic cholinergic stimulus-response coupling. Biochem Soc Trans. 1977;5(1):77–81. doi: 10.1042/bst0050077. [DOI] [PubMed] [Google Scholar]
- Mongar J. L., Svec P. The effect of phospholipids on anaphylactic histamine release. Br J Pharmacol. 1972 Dec;46(4):741–752. doi: 10.1111/j.1476-5381.1972.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron Y., Löwe M., Selinger Z. Incorporation of inorganic [32P] phosphate into rat parotid phosphatidylinositol. Induction through activation of alpha adrenergic and cholinergic receptors and relation to K+ release. Mol Pharmacol. 1975 Jan;11(1):79–86. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SAEKI K. EFFECTS OF COMPOUND 48/80, CHYMOTRYPSIN AND ANTI-SERUM ON ISOLATED MAST CELLS UNDER AEROBIC AND ANAEROBIC CONDITIONS. Jpn J Pharmacol. 1964 Sep;14:375–390. doi: 10.1254/jjp.14.375. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Sasaki J. Mechanism of histamine release by alpha-chymotrypsin from isolated rat mast cells. Jpn J Pharmacol. 1975 Jun;25(3):311–324. doi: 10.1254/jjp.25.311. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UVNAES B., ANTONSSON J. TRIGGERING ACTION OF PHOSPHATIDASE A AND CHYMOTRYPSINS ON DEGRANULATION OF RAT MESENTERY MAST CELLS. Biochem Pharmacol. 1963 Aug;12:867–873. doi: 10.1016/0006-2952(63)90117-5. [DOI] [PubMed] [Google Scholar]


