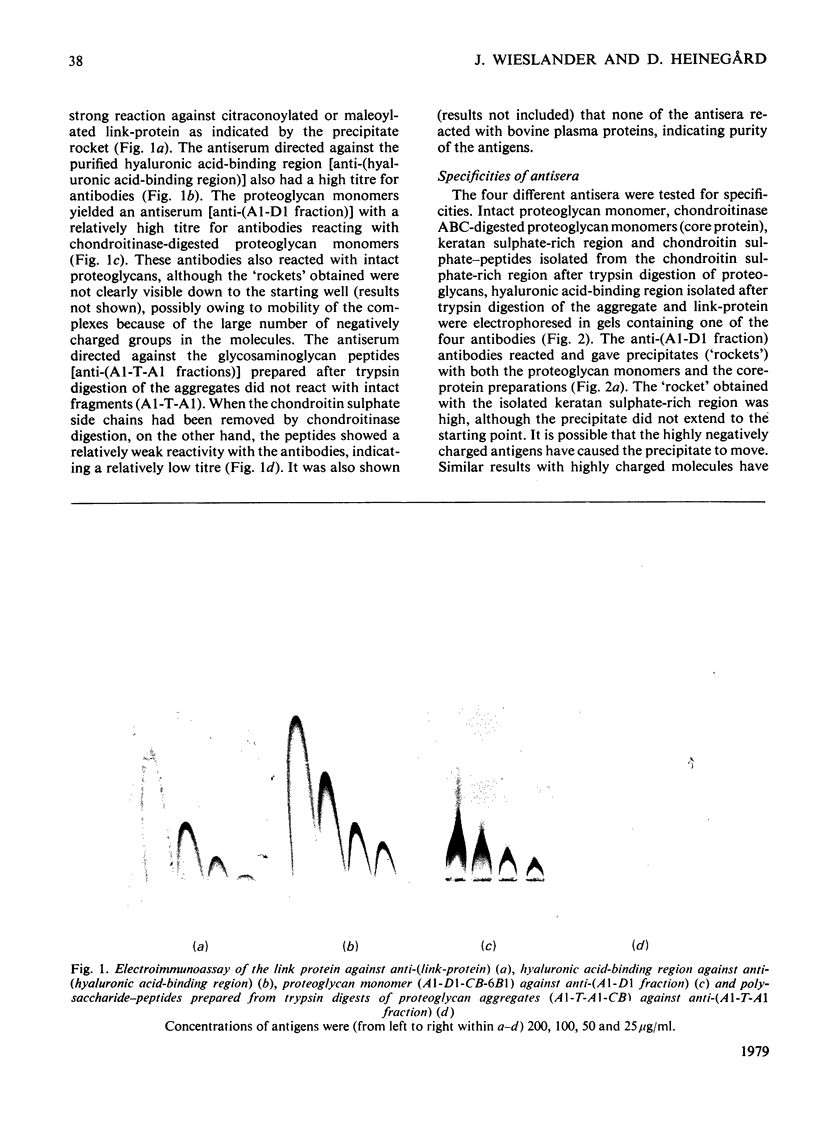
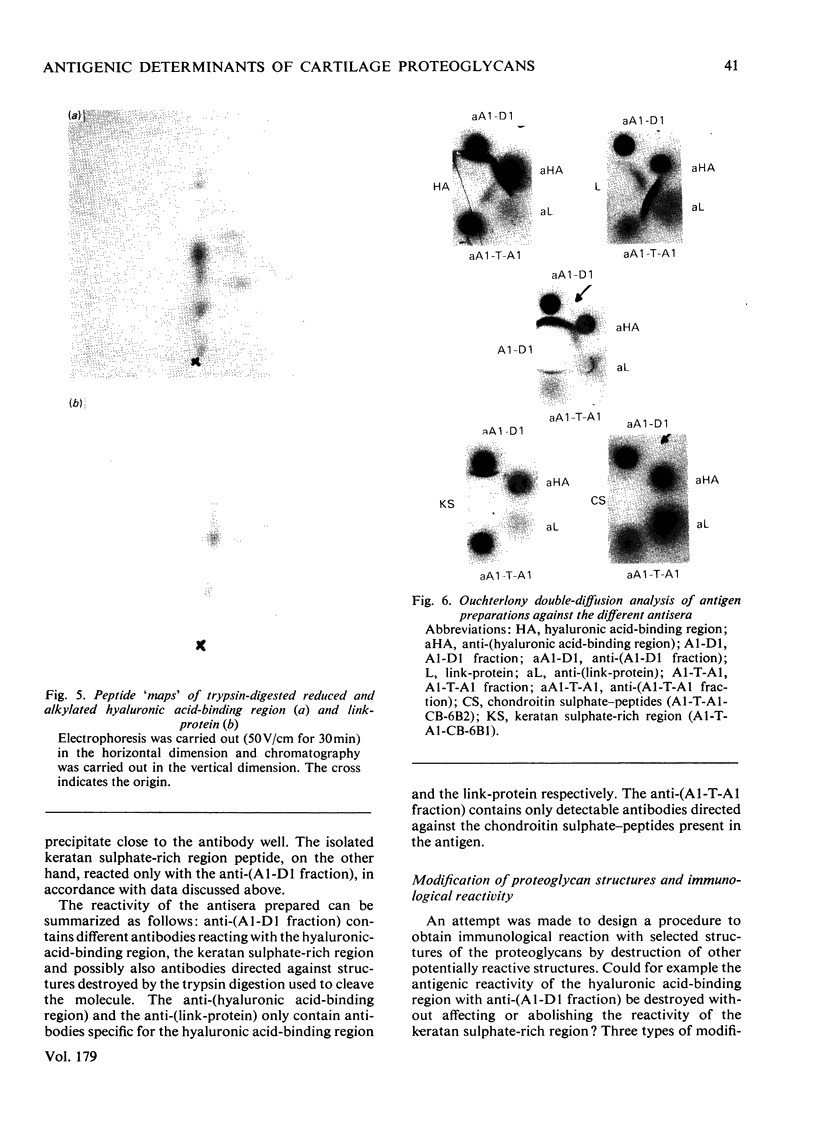
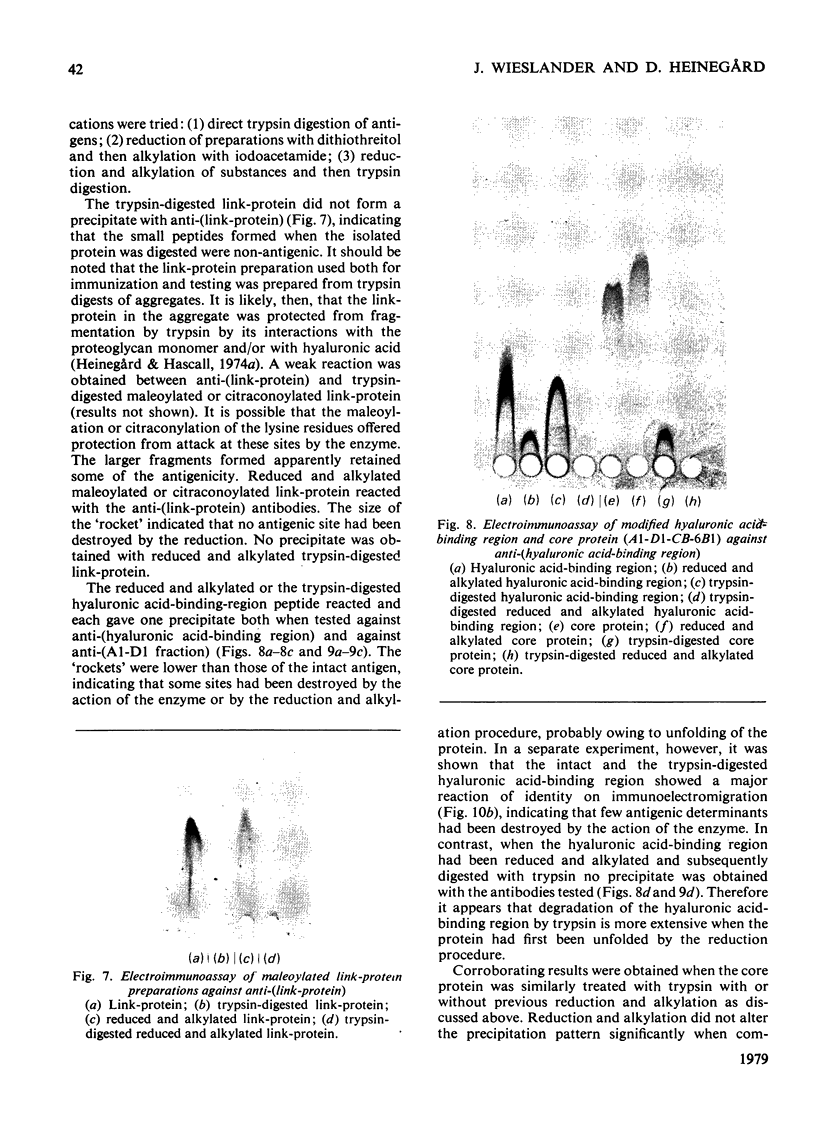
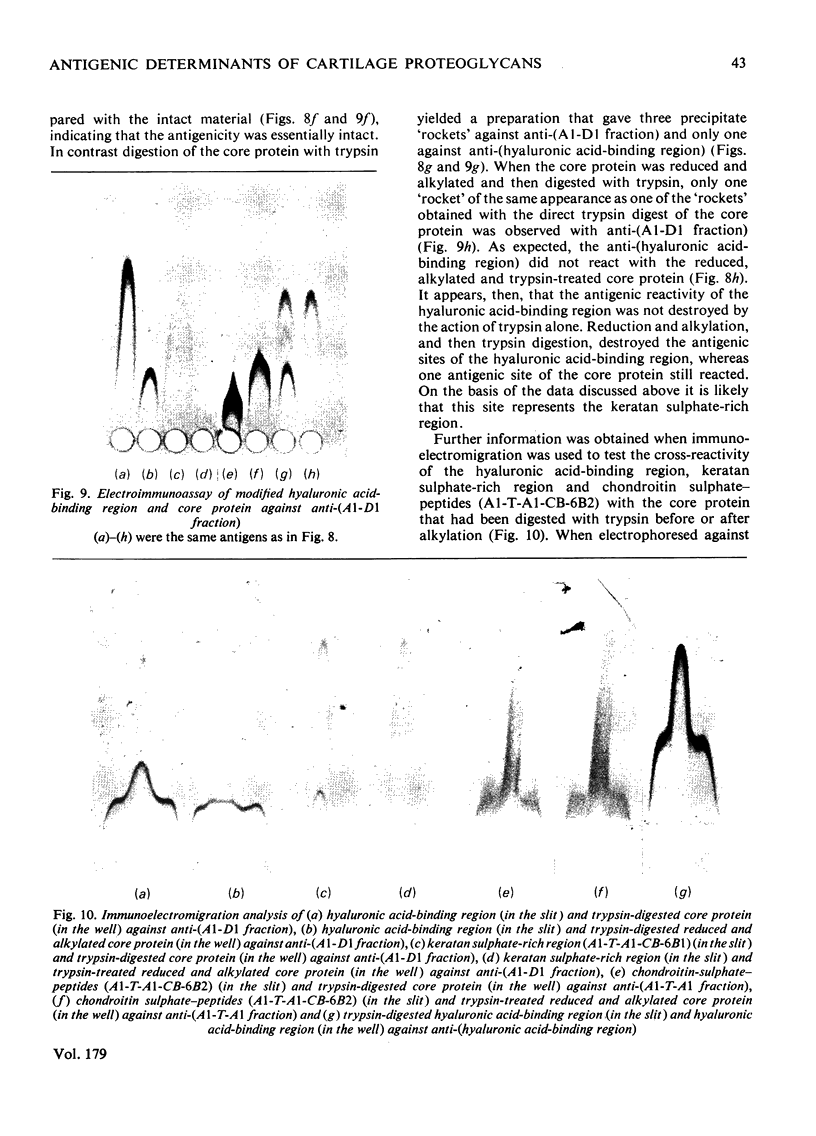
Abstract
Antibodies were raised in rabbits by injection of cartilage proteoglycan monomers, isolated hyaluronic acid-binding region, polysaccharide-peptides prepared by trypsin digestion of proteoglycans and link-protein. The rabbits injected with the proteoglycan monomers made antibodies reacting with the intact proteoglycan. The antiserum contained antibodies specific for, and also reacting with, the isolated hyaluronic acid-binding region and the keratan sulphate-rich region. In addition there were probably antibodies reacting with other structures of the proteoglycan monomer. When isolated hyaluronic acid-binding region was used for immunization the antibodies obtained reacted specifically with the hyaluronic acid-binding region. The antibodies obtained from rabbits immunized with the polysaccharide-peptides reacted with the proteoglycan monomers and showed a reaction identical with that of the chondroitin sulphate-peptides isolated after trypsin digestion of proteoglycans. The antibodies prepared with the link-protein as the antigen reacted only with the link-protein and not with any preparation from the proteoglycan monomer. Neither did any of the antisera raised against the proteoglycan monomer or its substructures react with the link-protein. Separately it was shown that the peptide 'maps' prepared from trypsin digests of the link-protein and the hyaluronic acid-binding region were different. Therefore it appears that the link-protein is not structurally related to the proteoglycan or the hyaluronic acid-binding region. Digestion of proteoglycan monomers or isolated hyaluronic acid-binding region with trypsin did not destroy the antigenic sites of the hyaluronic acid-binding region. In contrast trypsin digests of previously reduced and alkylated preparations did not react with the anti-(hyaluronic acid-binding region). The trypsin digests, however, reacted with both the antibodies directed against the chondroitin sulphate-peptides and those against the keratan sulphate-peptides. Trypsin digestion of the link-proteins destroyed the antigenic site and the reactivity with the antibodies. By combining immunoassay of proteoglycan preparations before and after trypsin digestion it is feasible to quantitatively determine its substructures by using the antisera described above.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Caterson B., Baker J. The interaction of link proteins with proteoglycan monomers in the absence of hyaluronic acid. Biochem Biophys Res Commun. 1978 Feb 14;80(3):496–503. doi: 10.1016/0006-291x(78)91596-6. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Keiser H., DeVito J. Immunochemical studies of fragments of bovine nasal cartilage proteoglycan subunit. Connect Tissue Res. 1974;2(4):273–282. doi: 10.3109/03008207409152256. [DOI] [PubMed] [Google Scholar]
- Keiser H. Immunological studies of the fragments of bovine cartilage proteoglycan produced by chondroitinase-trypsin digestion. Arch Biochem Biophys. 1975 Jun;168(2):622–629. doi: 10.1016/0003-9861(75)90294-5. [DOI] [PubMed] [Google Scholar]
- Keiser H., Shulman H. J., Sandson J. I. Immunochemistry of cartilage proteoglycan. Immunodiffusion and gel-electrophoretic studies. Biochem J. 1972 Jan;126(1):163–169. doi: 10.1042/bj1260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Loewi G., Muir H. The antigenicity of chondromucoprotein. Immunology. 1965 Aug;9(2):119–127. [PMC free article] [PubMed] [Google Scholar]
- Mason R. M., Mayes R. W. Extraction of cartilage protein-polysaccharides with inorganic salt solutions. Biochem J. 1973 Mar;131(3):535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) articular cartilage. Biochem J. 1977 Apr 1;163(1):103–109. doi: 10.1042/bj1630103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Lohmander S., Heinegård D. Proteoglycans of hyaline cartilage: Electron-microscopic studies on isolated molecules. Biochem J. 1975 Oct;151(1):157–166. doi: 10.1042/bj1510157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Bygren P., Heinegård D. Determination of the Tamm and Horsfall glycoprotein in human urine. Clin Chim Acta. 1977 Aug 1;78(3):391–400. doi: 10.1016/0009-8981(77)90072-9. [DOI] [PubMed] [Google Scholar]