Abstract
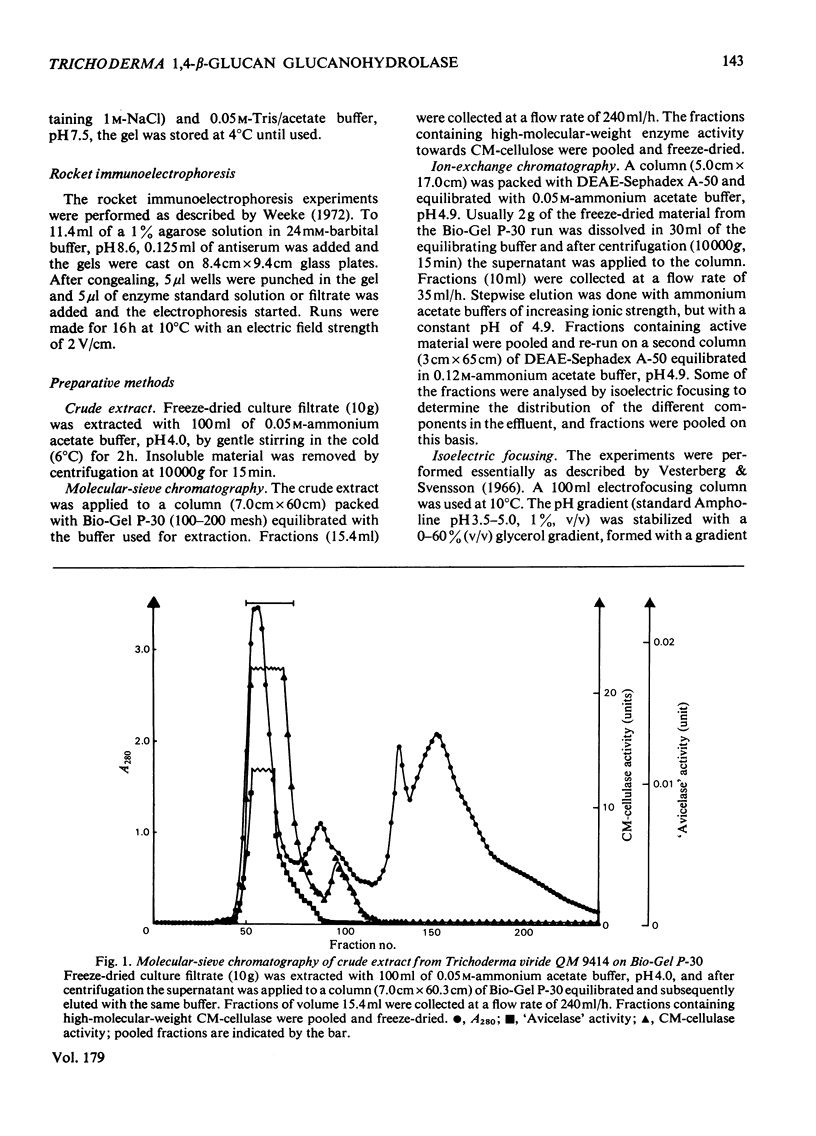
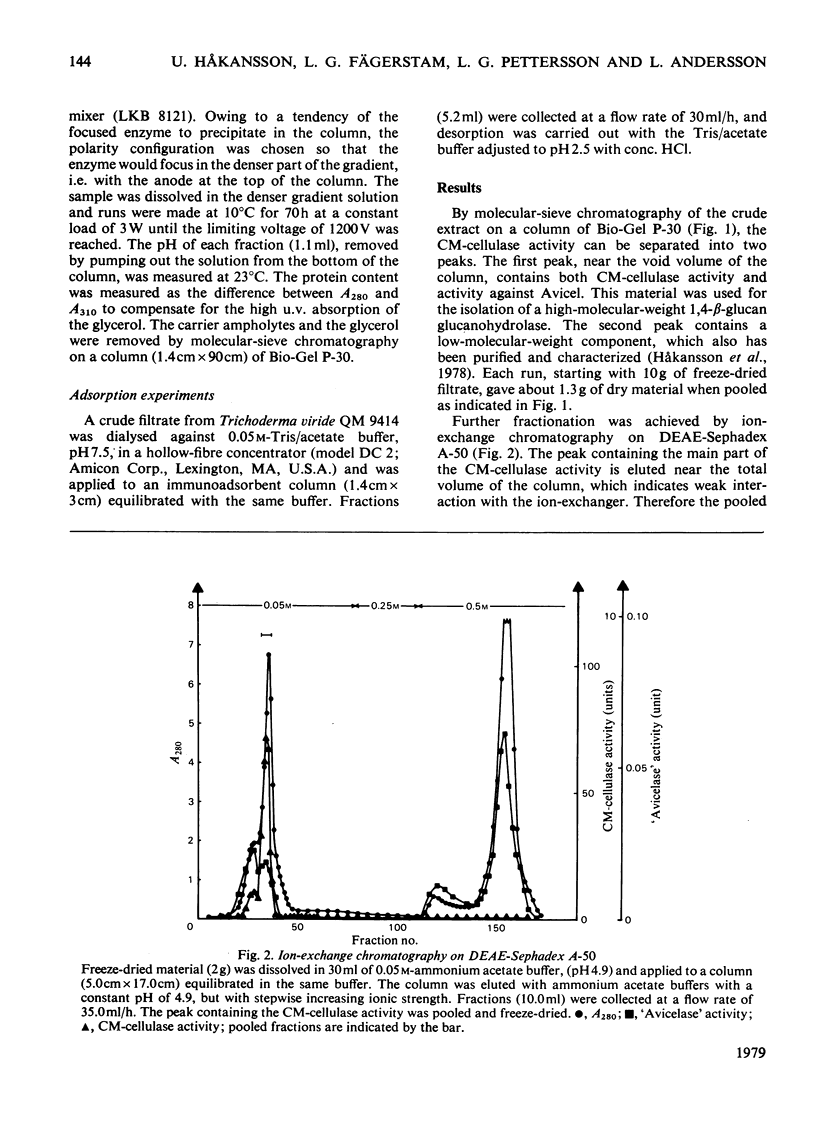
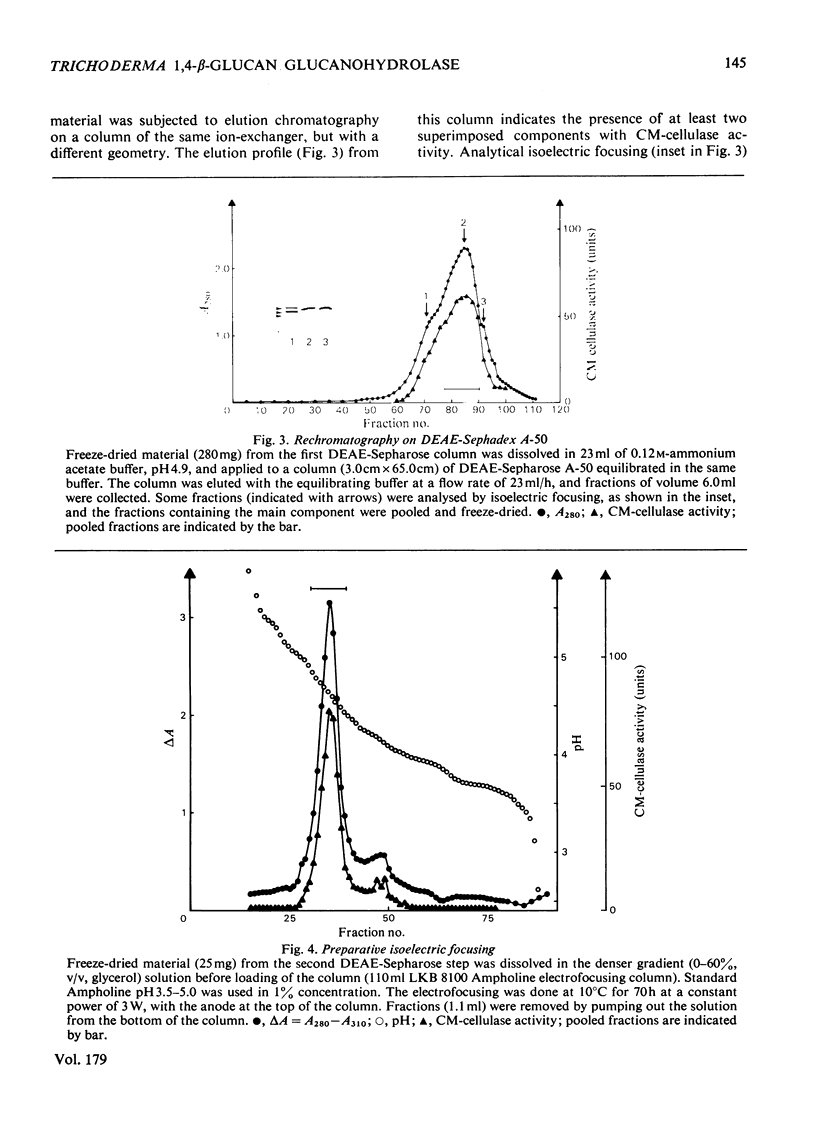
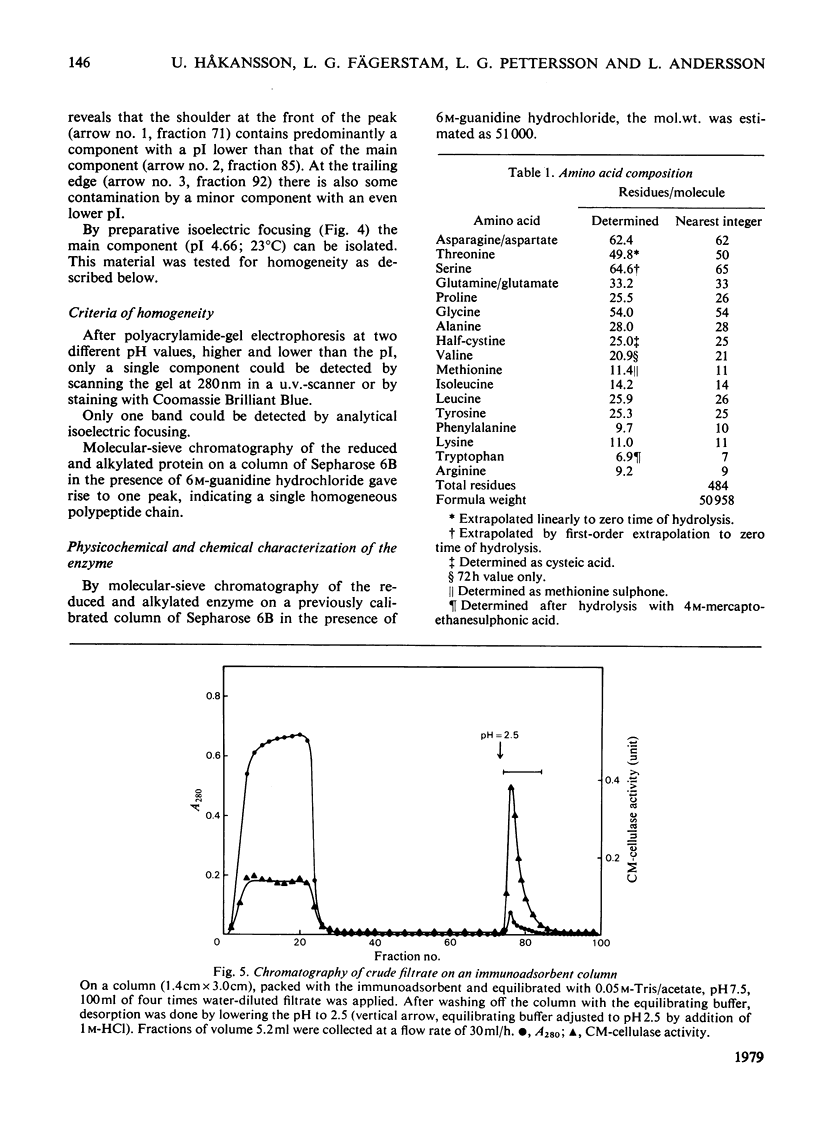
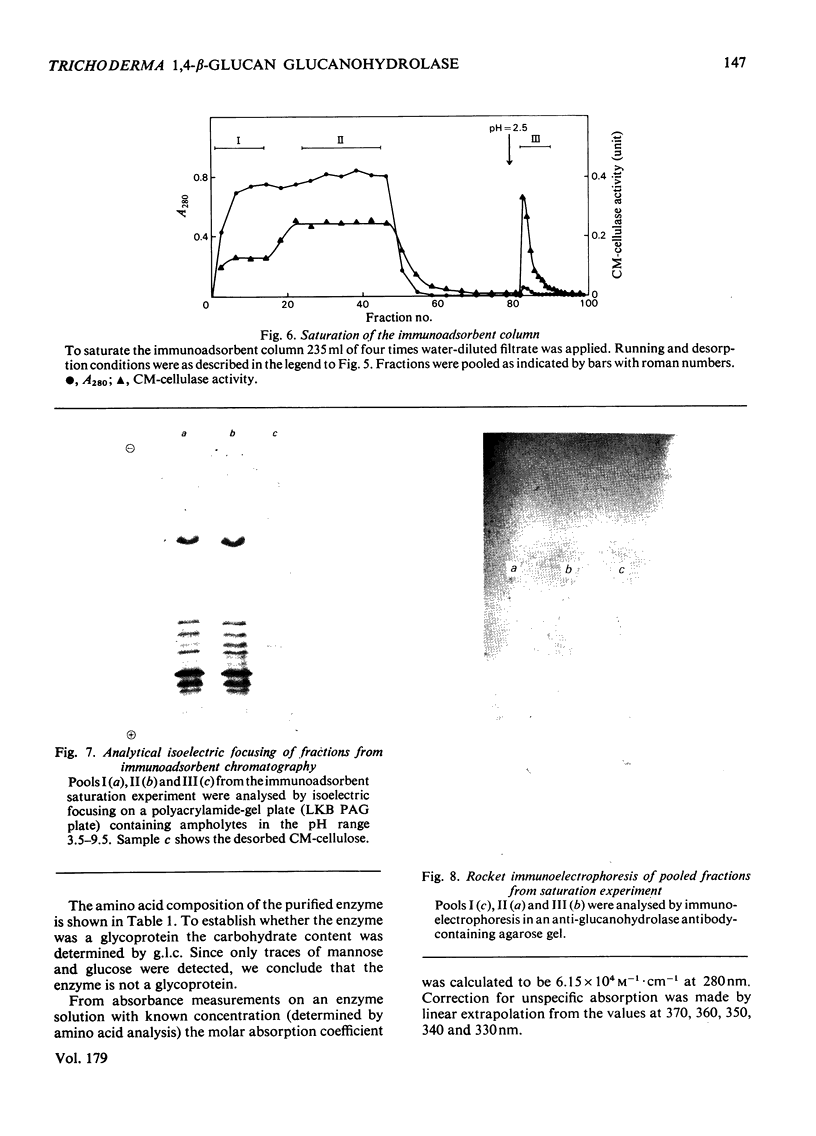
A 1,4-beta-glucan glucanohydrolase (EC 3.2.1.4) was isolated from culture filtrates of the fungus Trichoderma viride QM 9414 by molecular-sieve chromatography on Bio-Gel P-30, ion-exchange chromatography on DEAE-Sephadex A-50 and isoelectric focusing in a density gradient. Polyacrylamide-gel electrophoresis at two different pH values, analytical isoelectric focusing in a polyacrylamide-gel slab and molecular-sieve chromatography of the reduced and alkylated enzyme in a denaturing medium indicated a homogeneous protein. The enzyme has a mol.wt. of 51,000 and is not a glycoprotein. The pI was found to be 4.66 at 23 degrees C. Antiserum against the purified enzyme was prepared and the amount of enzyme in the original filtrate was determined by rocket immunoelectrophoresis to be about 50mg/liter. An immunoadsorbent made from CNBr-activated sepharose 4B and antiserum affords a rapid and highly specific purification of the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghem L. E., Pettersson L. G., Axiö-Fredriksson U. B. The mechanism of enzymatic cellulose degradation. Purification and some properties of two different 1,4beta-glucan glucanohydrolases from Trichoderma viride. Eur J Biochem. 1976 Jan 15;61(2):621–630. doi: 10.1111/j.1432-1033.1976.tb10058.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Fries E., Hjertén S. Scanning of polyacrylamide gel electrophoresis columns for detection of unstained protein zones and for their localization relative to enzyme activities. Anal Biochem. 1975 Apr;64(2):466–476. doi: 10.1016/0003-2697(75)90456-x. [DOI] [PubMed] [Google Scholar]
- Halliwell G., Riaz M. The formation of short fibres from native cellulose by components of Trichoderma koningii cellulase. Biochem J. 1970 Jan;116(1):35–42. doi: 10.1042/bj1160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjertén S., Jerstedt S., Tiselius A. Some aspects of the use of "continuous" and "discontinuous" buffer systems in polyacrylamide gel electrophoresis. Anal Biochem. 1965 May;11(2):219–223. doi: 10.1016/0003-2697(65)90008-4. [DOI] [PubMed] [Google Scholar]
- Håkansson U., Fägerstam L., Pettersson G., Andersson L. Purification and characterization of a low molecular weight 1,4-beta-glucan glucanohydrolase from the cellulolytic fungus Trichoderma viride QM 9414. Biochim Biophys Acta. 1978 Jun 9;524(2):385–392. doi: 10.1016/0005-2744(78)90175-4. [DOI] [PubMed] [Google Scholar]
- Jordan E. M., Raymond S. Gel electrophoresis: a new catalyst for acid systems. Anal Biochem. 1969 Feb;27(2):205–211. doi: 10.1016/0003-2697(69)90024-4. [DOI] [PubMed] [Google Scholar]
- Li L. H., Flora R. M., King K. W. Individual roles of cellulase components derived from Trichoderma viride. Arch Biochem Biophys. 1965 Aug;111(2):439–447. doi: 10.1016/0003-9861(65)90207-9. [DOI] [PubMed] [Google Scholar]
- Mandels M., Weber J., Parizek R. Enhanced cellulase production by a mutant of Trichoderma viride. Appl Microbiol. 1971 Jan;21(1):152–154. doi: 10.1128/am.21.1.152-154.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada G. Enzymatic studies on a cellulase system of Trichoderma viride. II. Purification and Properties of two cellulases. J Biochem. 1975 Jan 1;77(1?):33–42. [PubMed] [Google Scholar]
- Okada G., Nisizawa K., Suzuki H. Cellulase components from Trichoderma viride. J Biochem. 1968 May;63(5):591–607. doi: 10.1093/oxfordjournals.jbchem.a128818. [DOI] [PubMed] [Google Scholar]
- SELBY K., MAITLAND C. C. THE FRACTIONATION OF MYROTHECIUM VERRUCARIA CELLULASE BY GEL FILTRATION. Biochem J. 1965 Mar;94:578–583. doi: 10.1042/bj0940578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Selby K., Maitland C. C. The cellulase of Trichoderma viride. Separation of the components involved in the solubilization of cotton. Biochem J. 1967 Sep;104(3):716–724. doi: 10.1042/bj1040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Wood T. M. Cellulolytic enzyme system of Trichoderma koningii. Separation of components attacking native cotton. Biochem J. 1968 Sep;109(2):217–227. doi: 10.1042/bj1090217. [DOI] [PMC free article] [PubMed] [Google Scholar]