Abstract
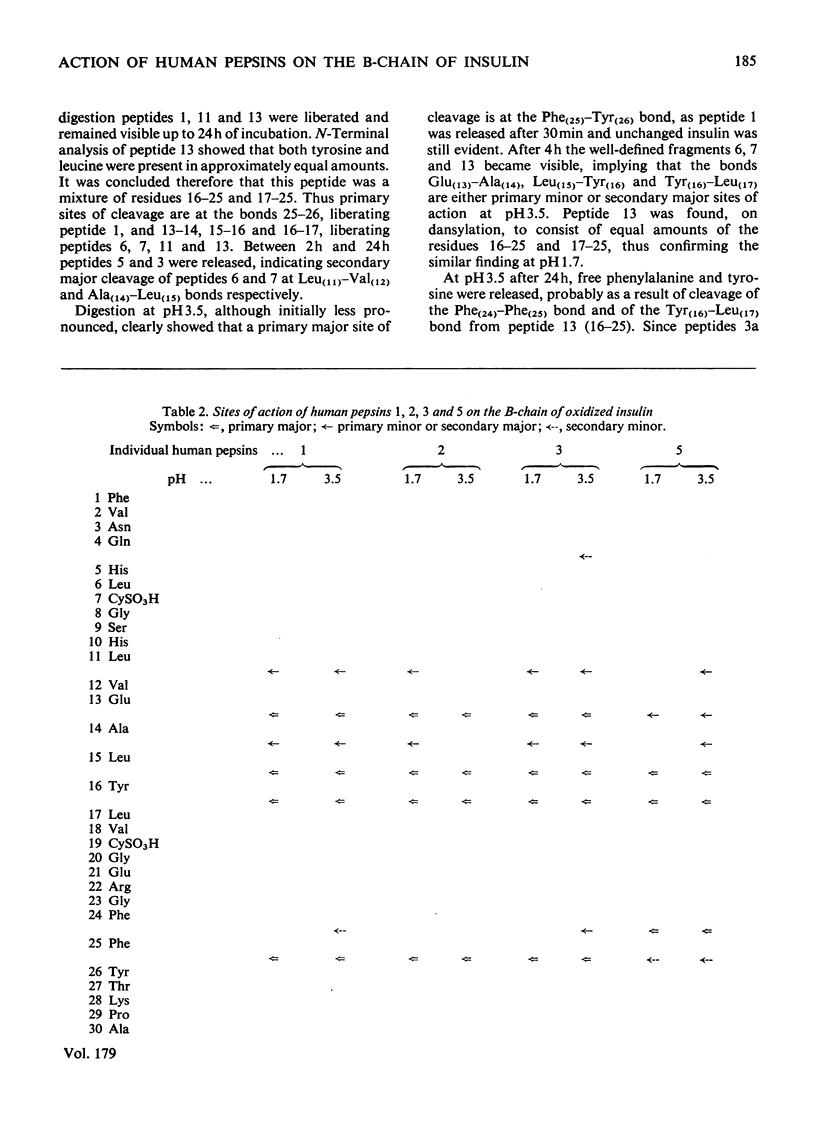
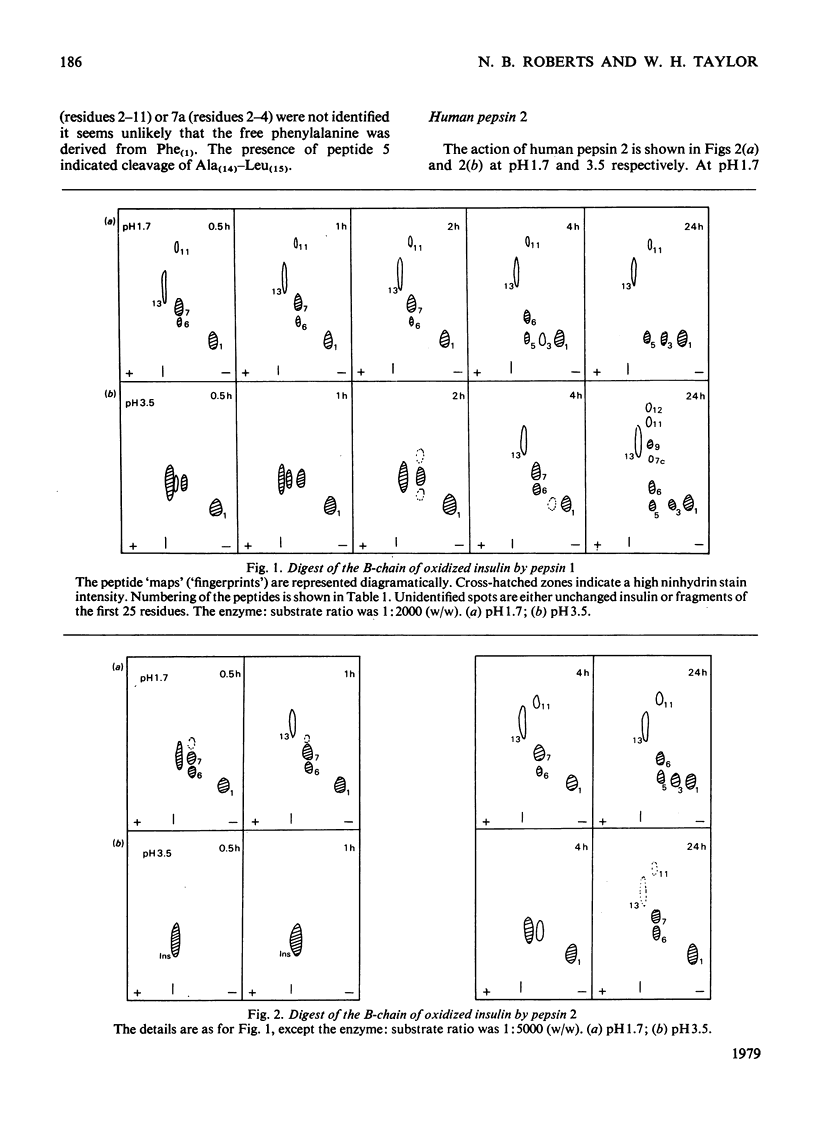
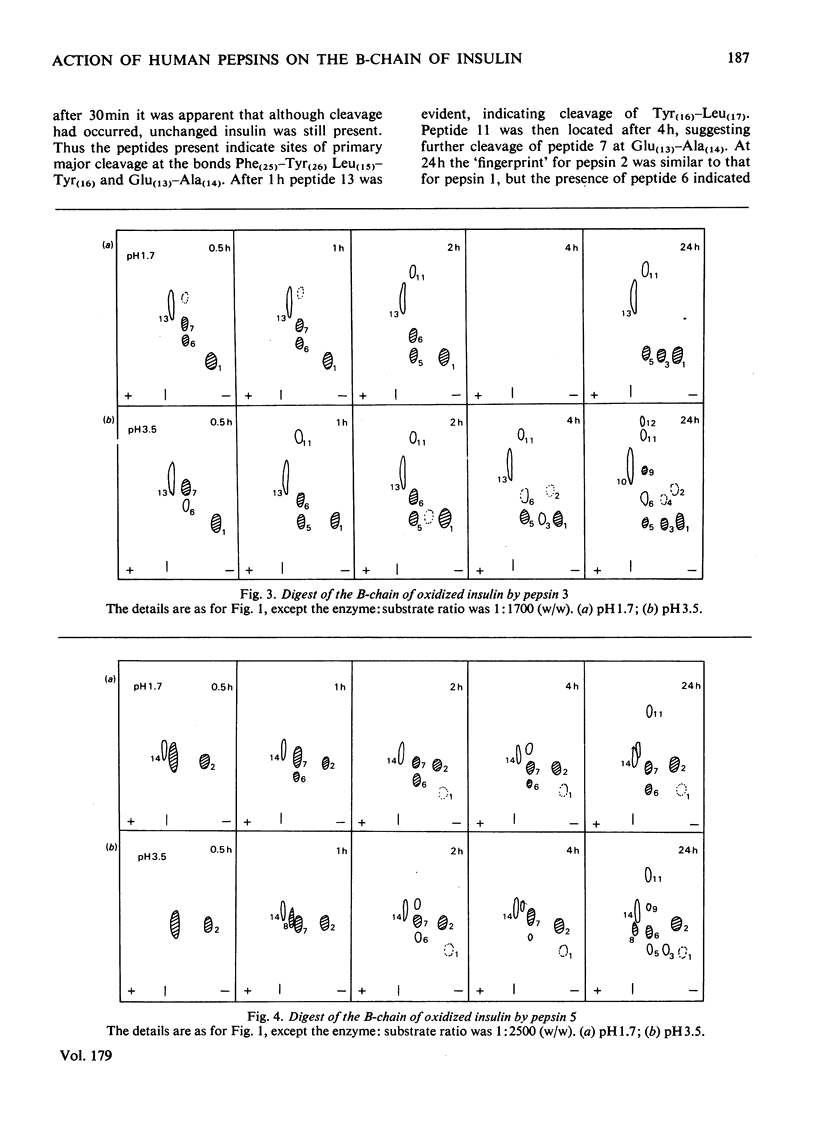
Human pepsins 1 and 2 attack the B-chain of oxidized insulin at pH 1.7 at the same bonds as does human pepsin 3. At pH 3.5, pepsins 1 and 2 attack insulin B-chain at essentially the same bonds as at pH 1.7, but more slowly. For all three enzymes, the first bond to be hydrolysed is Phe(25)-Tyr(26), followed simultaneously by Glu(13)-Ala(14), Leu(15)-Tyr(16) and Tyr(16)-Leu(17). Human pepsin 5, however, attacks Phe(24)-Phe(25) first of all, followed by Leu(15)-Tyr(16) and Tyr(16)-Leu(17). The results suggest that each pepsin has only one active site. Acid hydrolysis indicates that the sites of enzymic cleavage are not bonds with an inherent instability at low pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erlanger B. F., Vratsanos S. M., Wassermann N., Cooper A. G. Stereochemical investigation of the active center of pepsin using a new inactivator. Biochem Biophys Res Commun. 1967 Jul 21;28(2):203–208. doi: 10.1016/0006-291x(67)90430-5. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Taylor W. H. Nomenclature of the pepsins. Nature. 1967 Oct 21;216(5112):279–280. doi: 10.1038/216279a0. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Taylor W. H. The separation and preparation of human pepsins 3 and 5 from the gastric juice of single individuals, and the mode of action of human and swine pepsins on the B-chain of oxidized insulin. Biochim Biophys Acta. 1971 Apr 27;236(1):92–98. doi: 10.1016/0005-2795(71)90154-1. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hartsuck J. A., Tang J. The carboxylate ion in the active center of pepsin. J Biol Chem. 1972 Apr 25;247(8):2575–2580. [PubMed] [Google Scholar]
- Hill R. L. Hydrolysis of proteins. Adv Protein Chem. 1965;20:37–107. doi: 10.1016/s0065-3233(08)60388-5. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., PORTER R. R. Parapepsins: two proteolytic enzymes associated with porcine pepsin. Biochem J. 1959 Sep;73:75–86. doi: 10.1042/bj0730075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. B., Taylor W. H. The preparation and purification of individual human pepsins by using diethylaminoethyl-cellulose. Biochem J. 1978 Mar 1;169(3):607–615. doi: 10.1042/bj1690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochem J. 1951 Sep;49(4):481–490. doi: 10.1042/bj0490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. Fractionation of oxidized insulin. Biochem J. 1949;44(1):126–128. doi: 10.1042/bj0440126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H. Studies on gastric proteolysis. I. The proteolytic activity of human gastric juice and pig and calf gastric mucosal extracts below pH5. Biochem J. 1959 Jan;71(1):73–83. doi: 10.1042/bj0710073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. Specific and irreversible inactivation of pepsin by substrate-like epoxides. J Biol Chem. 1971 Jul 25;246(14):4510–4517. [PubMed] [Google Scholar]
- Taylor W. H. Pepsins of patients with peptic ulcer. Nature. 1970 Jul 4;227(5253):76–77. doi: 10.1038/227076a0. [DOI] [PubMed] [Google Scholar]


