Abstract
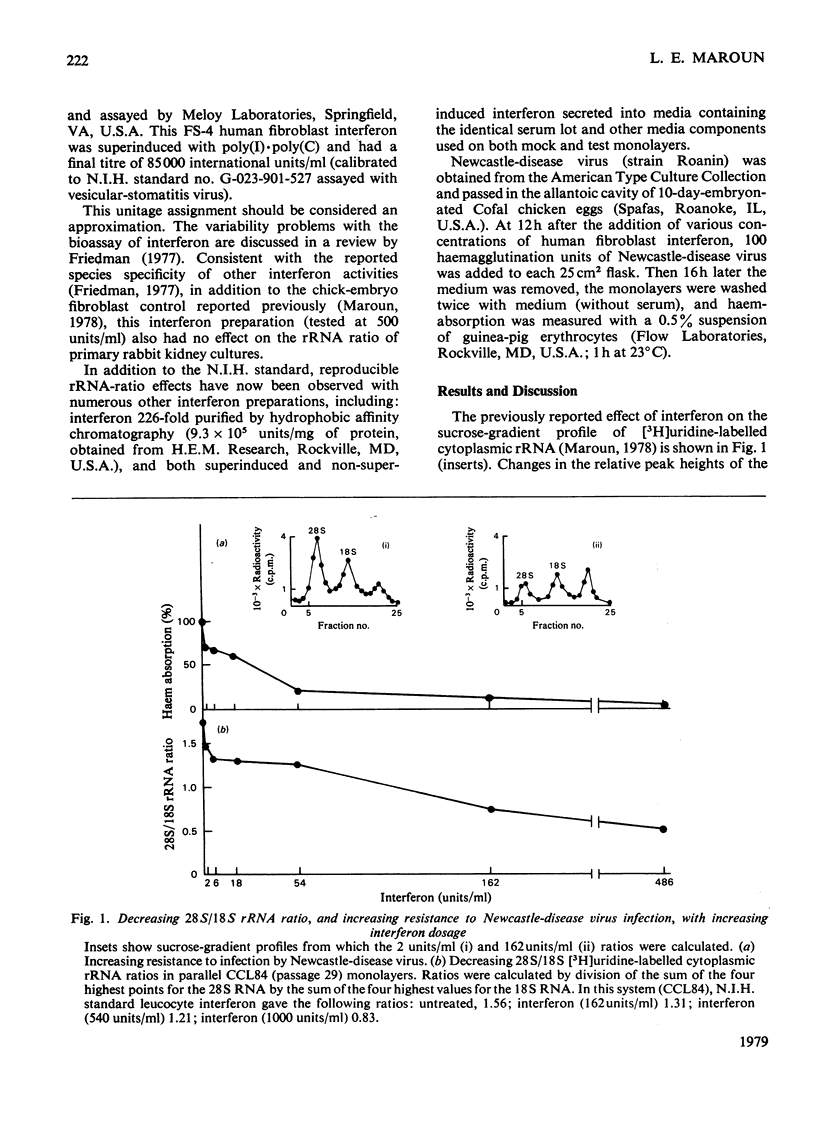
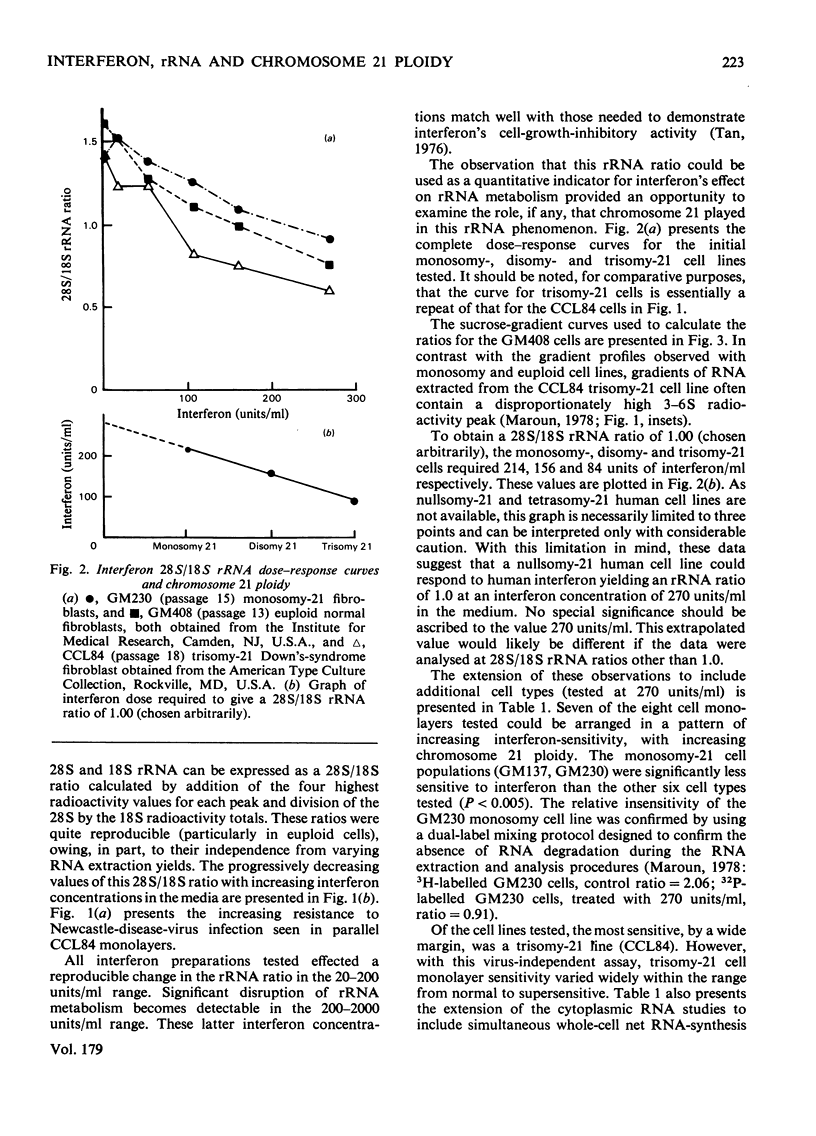
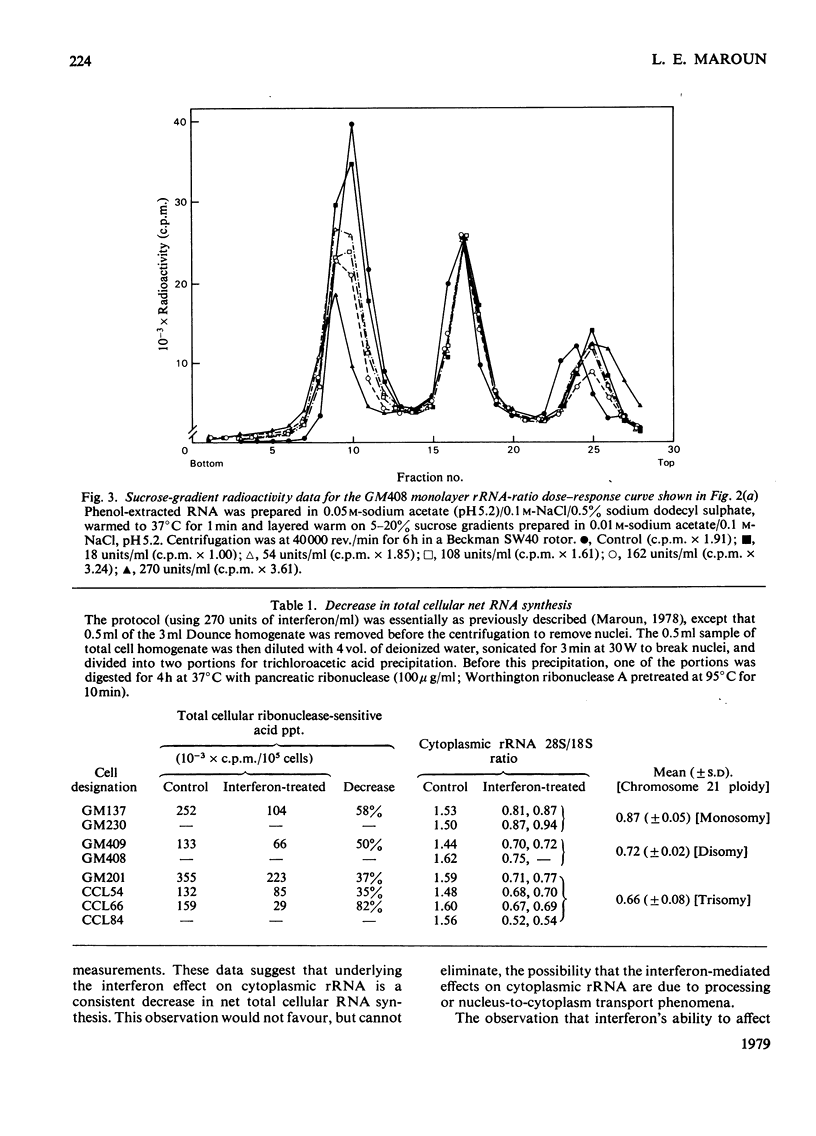
Antiviral and cell-growth-inhibitory activities of human interferon were shown to be related to the activity of a gene or genes present on chromosome 21. The 18s rRNA is vital to cell growth; it is capable of a viral-mRNA-recognition function and it is coded for by genes a portion of which are present on chromosome-21. A previously reported ability of human interferon to affect rRNA metabolism is characterized by a decrease in the sucrose-gradient-peak ratio of radiolabelled 28S to 18S rRNA in extracts from the cytoplasm of interferon-treated human fibroblasts. In the present report, interferon dose-response curves are presented demonstrating a direct relationship between a decrease in this ratio and interferon concentrations in the media. By using this virus-independent cytoplasmic rRNA assay, eight human fibroblast lines, differing in chromosome 21 ploidy, were tested for sensitivity to human interferon. Two monosomy-21, two euploid-21 and four trisomy-21 cell lines were tested. The monosomy-21 cell populations were significantly less sensitive to interferon than the other six cell types tested. Of the cell lines tested, the most sensitive, by a wide margin, was a trisomy-21 line. Trisomy-21 cell monolayer sensitivity, however, varied widely within the range from normal to supersensitive. These observations suggest that interferon's ability to affect rRNA metabolism is related to the activity of a gene or genes present on chromosome 21.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bross K., Dittes H., Krone W., Schmid M., Vogel W. Biochemical and cytogenetic studies on the nucleolus organizing regions (NOR) of man. I. Comparison of trisomy 21 with balanced translocations t(DqGq). Humangenetik. 1973 Dec 10;20(3):223–229. doi: 10.1007/BF00385734. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Edy V. G., Cassiman J. J. Chromosome 21 does not code for an interferon receptor. Nature. 1976 Nov 18;264(5583):249–251. doi: 10.1038/264249a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Edy V. G., Cassiman J. Non-antiviral activities of interferon are not controlled by chromosome 21. Nature. 1975 Jul 10;256(5513):132–134. doi: 10.1038/256132a0. [DOI] [PubMed] [Google Scholar]
- Dittes H., Krone W., Bross K., Schmid M., Vogel W. Biochemical and cytogenetic studies on the nucleolus organizing regions (NOR) of man. II. A family with the 15/21 translocation. Humangenetik. 1975;26(1):47–59. doi: 10.1007/BF00280284. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Potentiation of hemoglobin messenger ribonucleic acid. A step in protein synthesis initiation involving interaction of messenger with 18 S ribosomal ribonucleic acid. J Biol Chem. 1975 Aug 10;250(15):6085–6092. [PubMed] [Google Scholar]
- Maroun L. E., Driscoll B. F., Nardone R. M. Possible cytoplasmic precursor of haemoglobin messenger RNA. Nat New Biol. 1971 Jun 30;231(26):270–271. doi: 10.1038/newbio231270a0. [DOI] [PubMed] [Google Scholar]
- Maroun L. E. Interferon-mediated effect on ribosomal RNA metabolism. Biochim Biophys Acta. 1978 Jan 26;517(1):109–114. doi: 10.1016/0005-2787(78)90038-2. [DOI] [PubMed] [Google Scholar]
- Maroun L. E., Miller E. T. Increased messenger RNA from protein synthesis inhibited human fibroblasts. J Cell Physiol. 1977 Sep;92(3):375–379. doi: 10.1002/jcp.1040920306. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Tantravahi R., Dev V. G., Miller O. J. Frequency of satellite association of human chromosomes is correlated with amount of Ag-staining of the nucleolus organizer region. Am J Hum Genet. 1977 Sep;29(5):490–502. [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Miller D. A., Dev V. G., Tantravahi R., Croce C. M. Expression of human and suppression of mouse nucleolus organizer activity in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4531–4535. doi: 10.1073/pnas.73.12.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Bash D., Ruddle F. H. Antibodies to a cell-surface component coded by human chromosome 21 inhibit action of interferon. Nature. 1976 Mar 11;260(5547):139–141. doi: 10.1038/260139a0. [DOI] [PubMed] [Google Scholar]
- Stoller A., Collmann R. D. Incidence of infective hepatitis followed by Down's syndrome nine months later. Lancet. 1965 Dec 11;2(7424):1221–1223. doi: 10.1016/s0140-6736(65)90642-2. [DOI] [PubMed] [Google Scholar]
- Tan U. H. Chromosome-21-dosage effect on inducibility of anti-viral gene(s). Nature. 1975 Jan 24;253(5489):280–282. doi: 10.1038/253280a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H. Chromosome 21 and the cell growth inhibitory effect of human interferon preparations. Nature. 1976 Mar 11;260(5547):141–143. doi: 10.1038/260141a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Greene A. E. Subregional localization of the gene(s) governing the human interferon induced antiviral state in man. J Gen Virol. 1976 Jul;32(1):153–155. doi: 10.1099/0022-1317-32-1-153. [DOI] [PubMed] [Google Scholar]


