Abstract
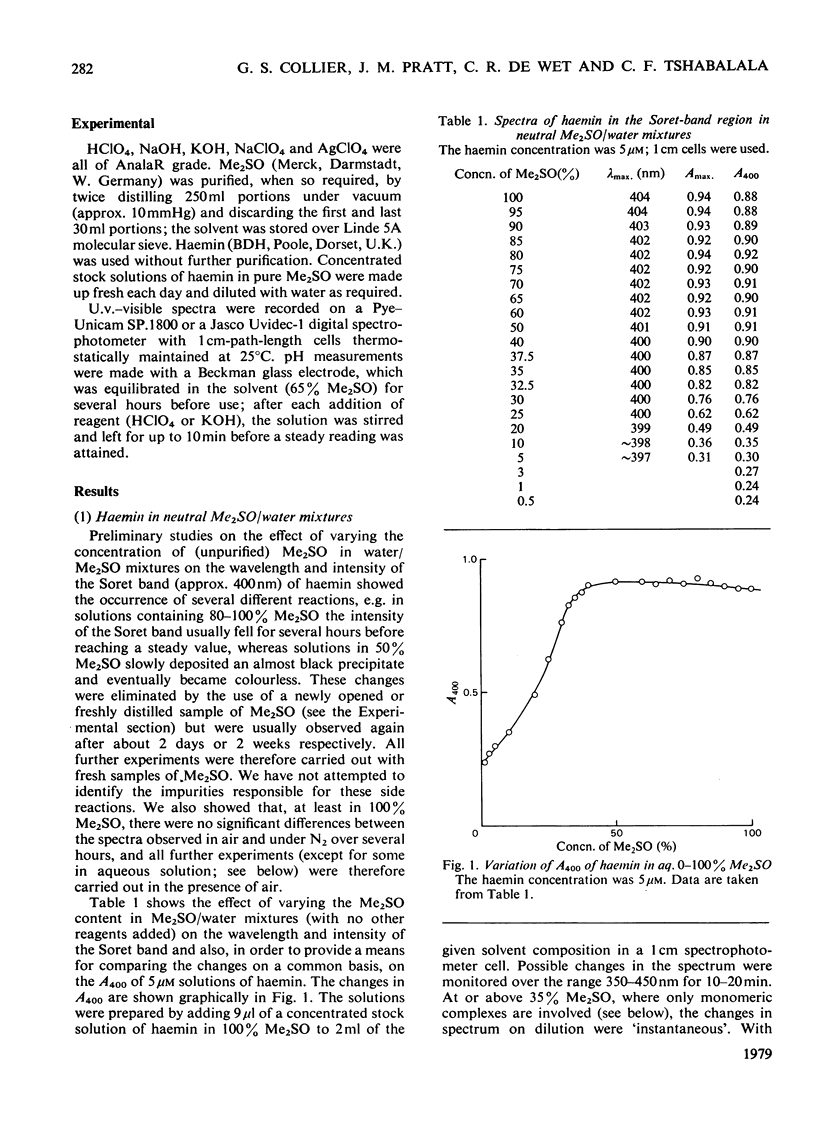
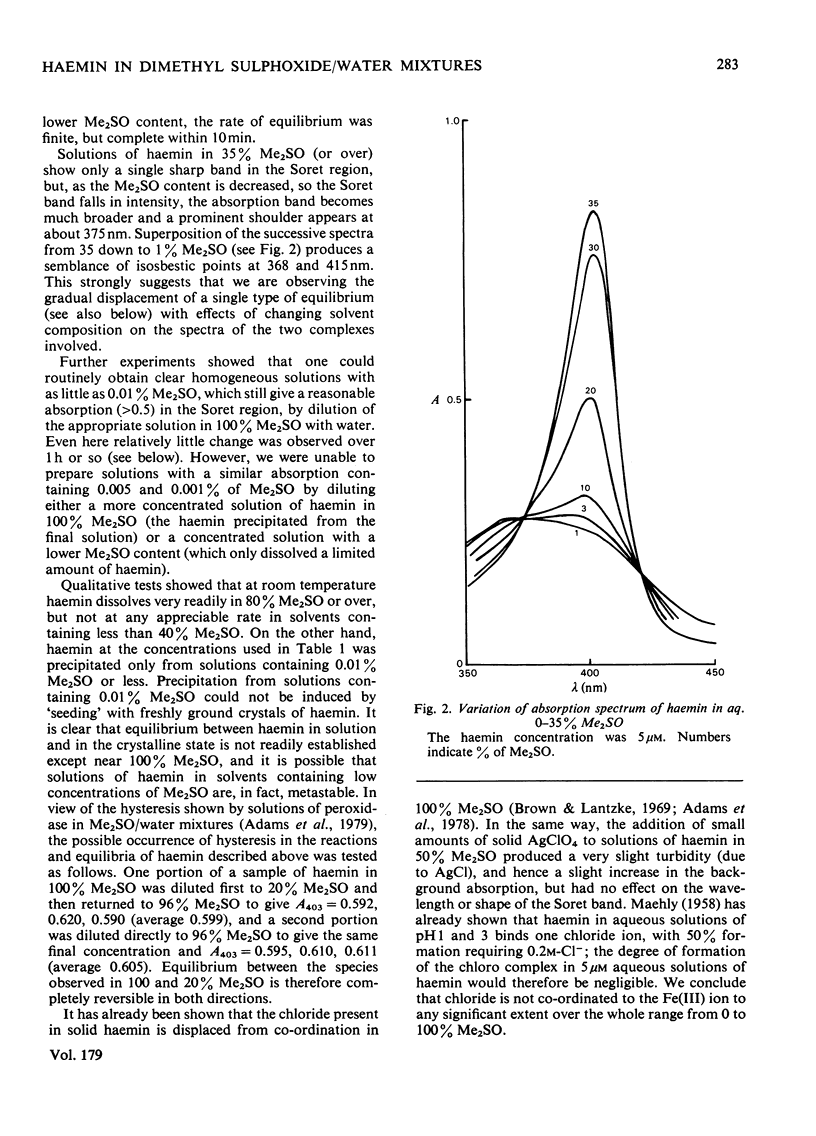
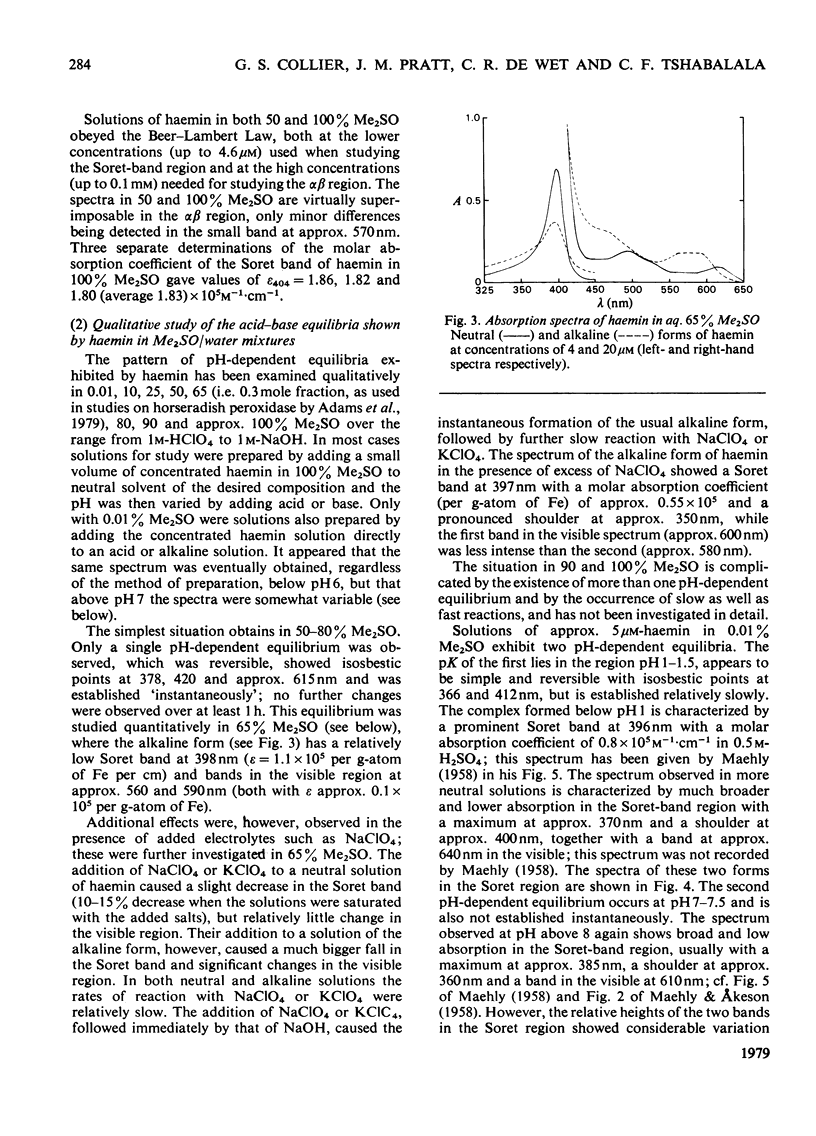
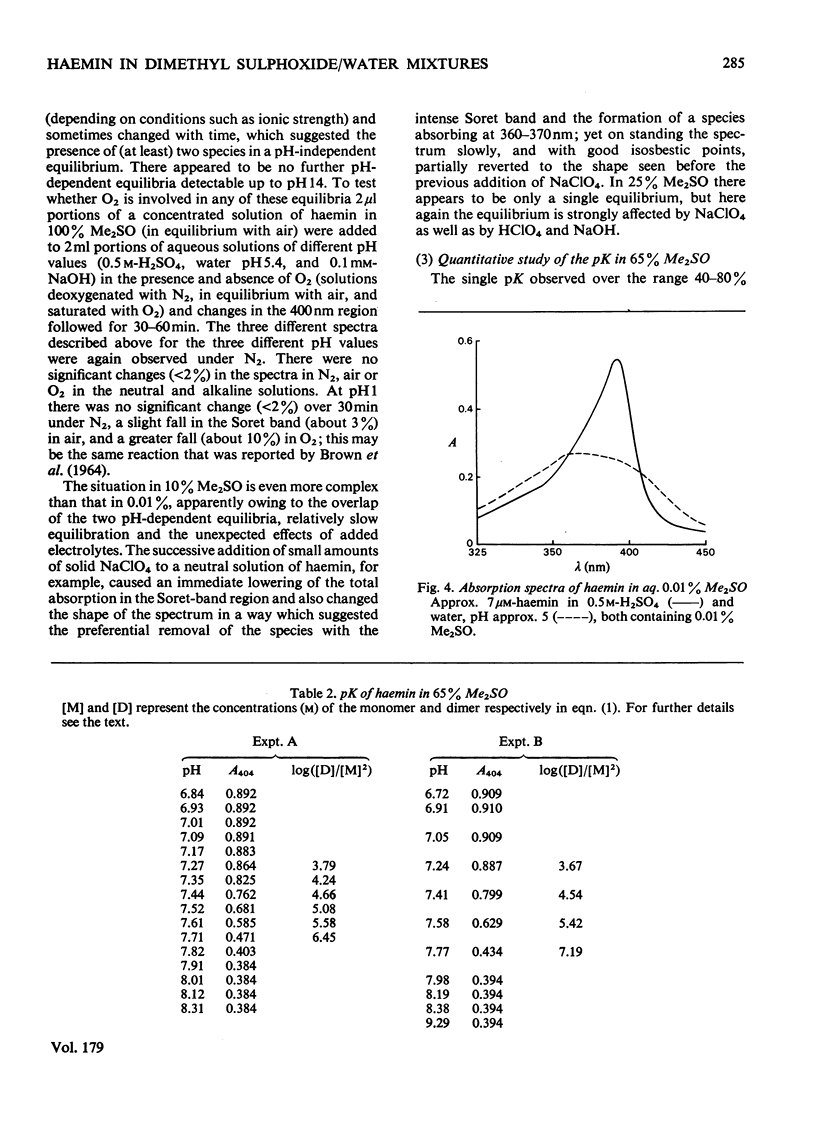
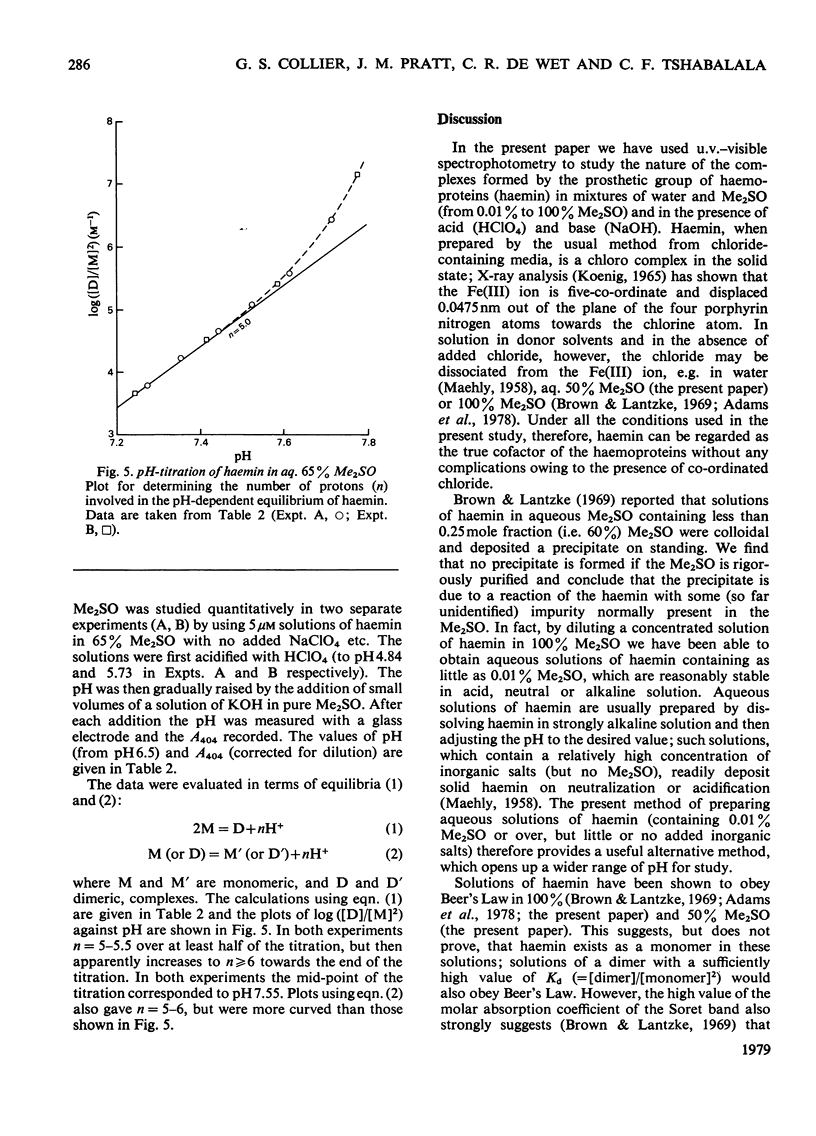
The nature of the complexes and equilibria shown by solutions of protohaemin in dimethyl sulphoxide/water mixtures and in the presence of acid and base were studied by u.v.-visible spectrophotometry. In neutral solutions containing from 40 to 100% dimethyl sulphoxide, haemin is present as a monomeric complex in which the Cl-ion is not coordinated. Only a single pH-dependent equilibrium pK12 is observed over the range 40-80% dimethylsulphoxide, corresponding to formation of the mu-oxo dimer. As the dimethyl sulphoxide content is lowered below 35%, so the single equilibrium (pK12) is replaced by two equilibria (pK1 and pK2); with solutions of 5 microM-haemin, pK1 decreases (from pK12 7.55 in 65% dimethyl sulphoxide to pK1 approx. 1.5 in 0.01% dimethyl sulphoxide), whereas pK2 hardly changes (from pK12 7.55 in 65% to pK2 approx. 7.5 in 0.01%).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. A., Baldwin D. A., Collier G. S., Pratt J. M. Studies on horseradish peroxidase in dimethyl sulphoxide/water mixtures. The activation of hydrogen peroxide and the binding of fluoride. Biochem J. 1979 May 1;179(2):273–280. doi: 10.1042/bj1790273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven G. H., Chen S. H., d' Albis A., Gratzer W. B. A spectroscopic study of the haemin--human-serum-albumin system. Eur J Biochem. 1974 Feb 1;41(3):539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J. 1970 May;117(4):733–739. doi: 10.1042/bj1170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Lantzke I. R. Solution structures of ferrihaem in some dipolar aprotic solvents and their binary aqueous mixtures. Biochem J. 1969 Nov;115(2):279–285. doi: 10.1042/bj1150279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INADA Y., SHIBATA K. The Soret band of monomeric hematin and its changes on polymerization. Biochem Biophys Res Commun. 1962 Oct 31;9:323–327. doi: 10.1016/0006-291x(62)90048-7. [DOI] [PubMed] [Google Scholar]
- KOENIG D. F. THE STRUCTURE OF ALPHA-CHLOROHEMIN. Acta Crystallogr. 1965 Apr 10;18:663–673. doi: 10.1107/s0365110x65001536. [DOI] [PubMed] [Google Scholar]
- O'Keeffe D. H., Barlow C. H., Smythe G. A., Fuchsman W. H., Moss T. H., Lilienthal H. R., Caughey W. S. Magnetic and spectroscopic probes for FeOFe linkages in hemin systems. Bioinorg Chem. 1975;5(2):125–147. doi: 10.1016/s0006-3061(00)80056-3. [DOI] [PubMed] [Google Scholar]
- SCHELER W. [On the influence of solvent and pH on the light absorption of protoporphyrin IX and protohemin with a comparison of the light absorption of ferrihemoproteins and a classification of the spectra of protoporphyrin derivatives]. Biochem Z. 1960;332:344–365. [PubMed] [Google Scholar]


